The Small Laccase from Streptomyces coelicolor
J. Dohnálek1,2,
T. Skálová1, L. H. Østergaard3, P. R.
Østergaard3 and Jindřich Hašek1
1Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, v.v.i., Heyrovského nám. 2, 16206 Praha 6, Czech Republic
2Institute of Physics, Academy of Sciences of the Czech Republic, v.v.i.,Cukrovarnická 10, 16200 Praha 6, Czech Republic
3Novozymes A/S, Brudelysvej 26, DK-2880 Bagsværd, Denmark
dohnalek@imc.cas.cz
Laccases are enzymes capable of oxidation of phenolic compounds with concomitant reduction of molecular oxygen to water. The so called “large laccases” have been studied extensively and both functional and structural data are available [1]. Large laccases comprise three domains and their active sites are localized within one protein chain. Laccases utilize one copper ion of type I at the oxidation site and a trinuclear copper cluster in the interior of the enzyme facilitates electron transfer from substrate to oxygen. The gene encoding for the two-domain small laccase (SLAC) has been found in Streptomyces coelicolor, a known antibiotic producing bacterium. Phylogenetic and sequence comparison studies indicated the possible structure arrangement of small laccases as trimers [2].
Recombinant enzyme characterization lead to results supporting the idea of both dimeric and trimeric functional forms. SLAC expressed in Aspergillus oryzae and purified by a two-step ion exchange protocol was subjected to crystallization experiments and the best crystal form for structural studies grew with PEG 550 MME as precipitant [3].
The structure of SLAC was solved by multiple wavelength anomalous dispersion method (MAD) with diffraction data collected at beamline BM-14 of the European Synchrotron Radiation Source in Grenoble. SLAC forms trimers with the trinuclear clusters localized between individual chains and with specific trimerization features (Fig. 1). The first three-dimensional structure of this type of enzyme explains the characterization results, confirms the theoretical predictions and brings new questions regarding the catalytic action [4].
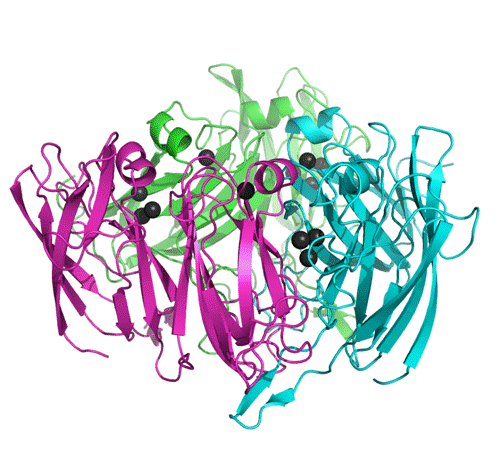
Figure 1. The Small Laccase trimer, secondary structure elements shown, chains distinguished by colour/ degree of grey, copper ions as spheres. Graphics created with program Pymol.
References
1. E. I. Solomon, U. M. Sundaram, T. E. Machonkin, Chem. Rev. 96, (1996), 2563.
2. K. Nakamura & N. Go, Cell. Mol. Life Sci. 62, (2005) 2050.
3. T. Skálová, J. Dohnálek, L.H.
Østergaard, P.R. Østergaard, P. Kolenko, J. Dušková, J. Hašek, Acta
Crystallogr., Sect. F, 63, (2007), 1077.
4. T. Skálová, J. Dohnálek, L.H.
Ostergaard, P.R. Ostergaard, P. Kolenko, J. Dušková, A. Štěpánková, J. Hašek, J. Mol. Biol., 385, (2009), 1165.
Acknowledgements.
This project was supported by the Ministry of Education, Youth and Sports of the Czech Republic (grant no. 1K05008) and by the Czech Science Foundation (grant no. 305/07/1073).