Cross-crystallization as a new optimization tool of crystallization
procedures
Ivana Tomčová1, 2 and Ivana Kutá Smatanová1, 2
1Institute
of Physical Biology, University of South Bohemia in České Budějovice,
Zámek 136, CZ-373 33 Nové Hrady, Czech Republic
2Institute
of Systems Biology and Ecology, Academy of Sciences of the Czech Republic,
Zámek 136, CZ-373 33 Nové Hrady, Czech Republic
tomcova@greentech.cz
Keywords: Crystal morphology; Single crystal growth; X-ray diffraction;
Biological macromolecules; Additives; Cupric compounds; Cross-crystallization
Abstract
The effect of several metal cations (Cu2+,
Cd2+, Co2+, Ba2+) was tested in attempts to
improve crystallization procedure and verify a newly discovered
cross-crystallization method with two selected proteins; di-heme cytochrome c4
from anaerobic purple sulphur bacterium Thiocapsa roseopersicina and
sweet-tasting protein thaumatin from the African berry Thaumatococcus
daniellii. Presence of Cu2+ ions promoted dramatic improvement
in crystal morphology, internal packing and diffraction quality. This
investigation qualitatively established the influence of cupric cations on the
crystal growth by using the cross-crystallization procedure. It was found that
influence of Cu2+ ions produced evidently different outer morphology
and internal packing of thaumatin crystals (hexagonal prism). Usually their
shape is presented as a tetragonal bipyramids. In the case of cytochrome, the
good diffractable crystals were obtained only by using cross-crystallization
method with metal-ion salts. Newly grown crystals (hexagonal prisms) of
thaumatin and cytochrome displayed as the same primitive tetragonal system and
diffracted up to 1.7 Å. Crystals were suitable for high-resolution
structure analysis.
Introduction
The determination of successful
crystallization conditions for a particular protein remains a highly empirical
process. Screening procedures are rapid and economical means to determine
preliminary crystallization conditions. During optimization a variable set of
parameters (i.e. pH, precipitant type, precipitant/protein concentration, etc.)
is screened to determine appropriate conditions for the nucleation and growth of
single crystals suitable for X-ray diffraction analysis. Unfortunately, in many
cases this strategy will not produce suitable single crystals. Empirically we have explored another
tool used in optimization strategy described by Tomčová [1]. We developed and
tested a crystallization procedure to modify crystal morphology, internal
packing and also to influence crystal growth. For the
first time the metal ion salts were added simultaneously to the protein drop
and even to neighbouring drops to allow cross-influence during crystallization
experiment. Here we report the effects of selected
additives on crystallization of two different proteins; one well-known “model”
protein thaumatin [2] and one crystallographicaly unexplored di-heme cytochrome
c4.
Methods
Description of cross-crystallization
method.
Cross-crystallization
is a procedure applied to standard vapor diffusion sitting and/or hanging drop
method. This procedure is based on using a set of additives that influence the
quality of crystal growth. In principle, the inclusion
of other droplets (containing chemical substances) against the same reservoir
slightly changes the vapor pressure of water over the neighboring drop
including protein. As described previously [1], the Emerald BioStructures
CombiClover Crystallization Plate (EBS plate, Emerald BioStructures, Bainbridge
Island WA, USA) with one central reservoir connected to four satellite
drop-chambers (a, b, c, d) via dedicated vapor diffusion channels, was used
in this procedure (Fig. 1a, 1b). Each of
drop-chambers a, b, c, d was filled with different additive (in this
case; chloride salts of copper, cadmium, cobalt and barium) and equal volume of
the precipitating agent. The protein was only added into the one drop-chamber
containing cupric chloride. Additives and reservoir solution, and not protein,
were placed to the three remaining drop-chambers to promote crystallization in
the fourth drop-chamber.
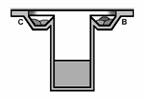
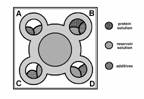
Fig. 1a, 1b: Schematic side and top view
of Emerald BioStructures CombiClover Crystallization Plate (EBS plate) for
sitting drop experiments. Grey color presents reservoir solution, strap areas
indicate each additives and grid represents protein-containing solution.
Results
Cross-crystallization experiments.
Cytochrome crystallization.
The cross-crystallization method was used
to further improve quality of crystals by addition of additives. Deep red
well-shaped cytochrome crystals grew within 3–4 days at 20 °C in the presence
of 5 mM cupric chloride and ammonium sulfate in citric acid buffer at pH 5.
Those crystals were not reproducible unless the other metal salts (CdCl2,
BaCl2, CoCl2) were present in the remaining drop chambers
as was described above. These cytochrome cross-crystallization experiments have
been tested several times, and in all cases the cytochrome crystals grew only
in hexagonal prism form. The same outer shape of crystals was observed when a
cytochrome was cross-crystallized by hanging drop (Fig. 2).
Thaumatin crystallization.
Thaumatin was crystallized using the
standard sitting drop method [2, 3] with the polyethylene glycol (PEG) as a
precipitating agent. Well-constructed tetragonal bipyramids were obtained from
these crystallization conditions. The effect of metal salt ions on
cross-crystallization was tested. Dramatic change in thaumatin crystal
morphology and internal packing was observed when thaumatin was crystallized as
hexagonal prisms (Fig. 2). In this case, cupric chloride caused the greatest change
in crystal outer shape while the other additives showed no significant effect
on crystal growth.
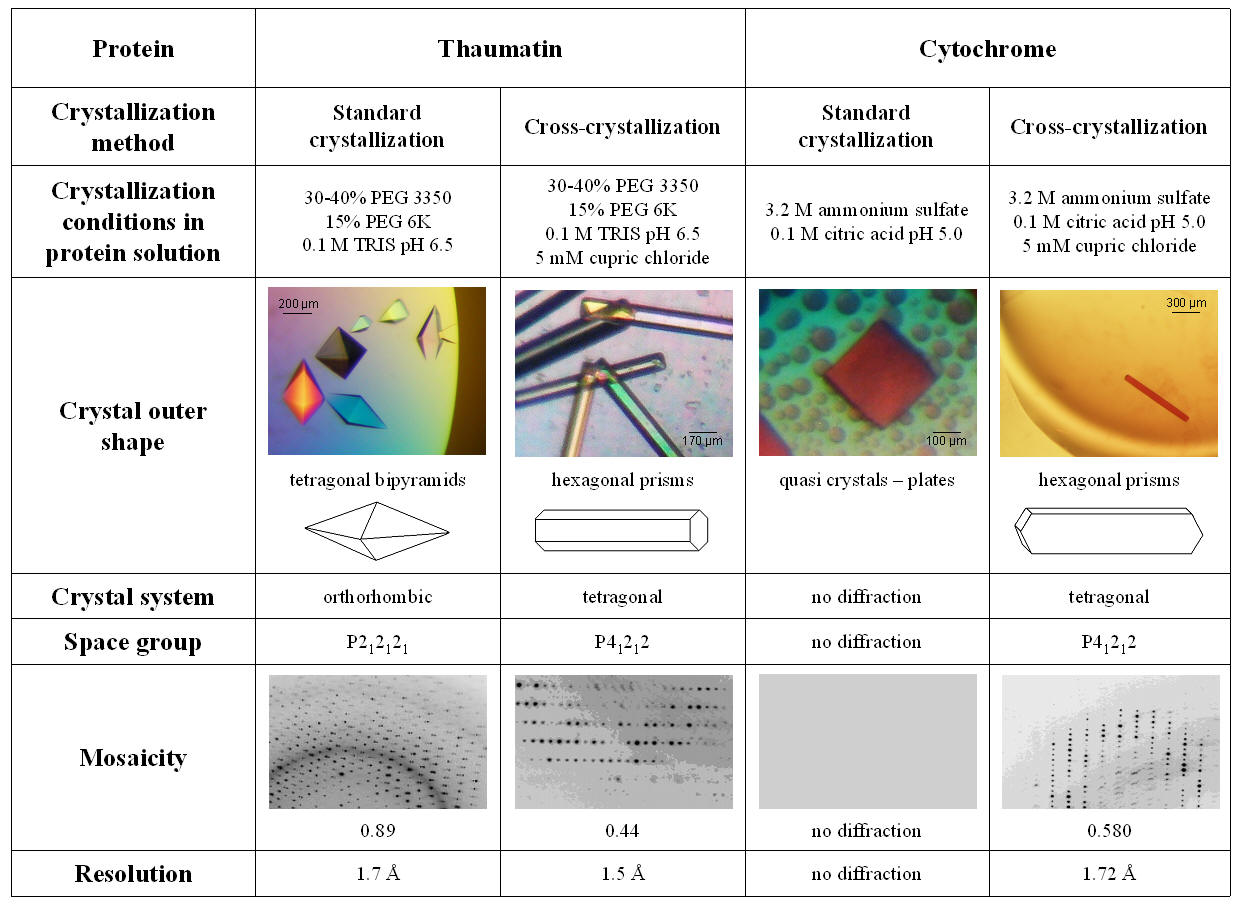
Fig. 2: Overview of thaumatin and cytochrome crystallization
experiments show crystal morphology and internal packing
influenced by metal-ion salts.
X-ray diffraction experiments.
Both, cytochrome and thaumatin hexagonal
prism crystals with dimensions of approximately 1.00 x 0.05 x 0.02 [mm] (Fig.
2) were tested at the synchrotron DESY/ EMBL. Complete data sets collections
were executed at beamline X13 with tunable wavelength using Oxford cryo-system
type magnets for crystal mounting. Crystals were removed from the drop with a
loop and flash-cooled in a nitrogen stream (Oxford cryo-system) at 100 K at the
goniometer part of beamline. A crystal to detector distance of 120 mm was used
to collect at least 200 frames of each. The exposure time for each image was 30
sec and the oscillation angle was 1°. Diffraction data were collected to 1.72
Å resolution for cytochrome, to 1.70 Å for thaumatin (tetragonal
bipyramid) and to 1.50 Å for thaumatin (hexagonal prism), using MAR CCD
165 mm detector at DORIS storage ring with triangular monochromator and bent
mirror beam.
Discussion
The
cross-crystallization method includes several factors that can facilitate
protein crystallization, from the promotion of intermolecular contacts by
divalent metal cations, stabilization of the protein with salts, to changing
the aggregation state with precipitating agents. In fact, any addition of a new
substance into a crystallizing mix resulting in crystallization is usually
classified as a new crystallization technique and handled as a hot tip. However,
the effectiveness of any newly discovered method could not be statistically
determined. For example, from previous studies it was found that cupric ions in
phosphate buffers have a tendency to produce heavy precipitate and even salt
crystals [4, 5]. Another example of an additive effect, which can be explained
on a molecular basis, is a formation of intermolecular contacts by intercalated
divalent transition metal cations [6]. Cadmium (in sulfate solutions) was long
known as a crystallization inducing agent of horse spleen ferritin and has been
re-discovered as a useful agent to promote crystallization or to increase
diffraction quality in a number of cases [Trakhanov 1998]. However, even with a
mechanistic explanation of this effect, no rational prediction regarding the
probability of success – except statistical evidence – is available!
The
specific morphology of thaumatin and cytochrome crystals may depend on factors
such as the source of material used during crystal growth and chemicals in the
crystallizing buffer in the mother liquor, or on the mother liquor itself. For
a single crystal form the angles between the faces are constant, but this is
not true if the crystals belong to the different crystal forms such as
tetragonal bipyramids and hexagonal prisms as in thaumatin. Their appearance
depends on the use of metal salt cations, such as cupric chloride, and
partially on the buffer and the precipitating agent used. We
assume these metal ions influence evaporation in the protein drop even if they
are absent from that drop. As this effect was tested on two different proteins
only, we cannot speculate about how universally applicable this will be. However,
the influence of Cu2+ ions on cytochrome crystal growth appears to
be specific, because no other successful combination of ion salts with
cytochrome was found among these four salts singly or in pairs. A similar
effect was observed even in thaumatin crystallization when conditions with
cupric chloride produced thaumatin crystals with a different morphology. The
combination of four particular salts that promote crystallization can be quite
reproducible also with other chemicals or even other volumes of the same drop
in the remaining drop chambers.
References
1.
I. Tomčová, R.M.M. Branca, G.
Bodó, Cs. Bagyinka, I. Kutá Smatanová, Acta Cryst. F62 (2006) 820-824.
2.
A. McPherson, Crystallization
of biological macromolecules, Cold Spring Harbor laboratory press, New York,
1999.
3.
T.M. Bergfors, Protein
crystallization: Techniques, strategies and tips, International University
Line, La Jolla, USA, 1999.
4.
A. McPherson, J. Weickmann, J.
Biomolecular Structure & Dynamics 7 (1990) 1053-1060.
5.
J. Jancarik, R. Pufan, C. Hong,
S.H. Kim, R. Kim, Acta. Crystal. Sec. D. D60 (2004) 1670-1673.
6.
H. Sigel, A. Sigel, Metal ions
in biological systems, Marcel Dekker - Taylor & Francis - CRC, California,
USA, 1990.
Acknowledgements.
This work is supported by grants
MSM6007665808 and LC06010 of the Ministry of Education of the Czech Republic and
Institutional research concept AVOZ60870520 of Academy of Sciences of the Czech
Republic to I.K.S.