Crystallization and structure - functional study of photosystem II from higher plants
Tatiana Prudnikovaa, Michal Kutýa, b, José A. Gavirac, Peter Palenčára, František Váchaa, e, Pavlína Řezáčovád, Juan M. García-Ruizc and Ivana Kutá Smatanováa, b
aInstitute of Physical Biology USB CB, Zamek 136, 373 33 Nove Hrady, Czech Republic
bInstitute of Systems Biology and Ecology AS CR Zamek 136, 373 33 Nove Hrady, Czech Republic
cLaboratorio de Estudios Cristalografico, Edf. Lopez Neira, P.T. Ciencias de la Salud, Avenida del Conocimiento, s/n, 18100 Armilla, Granada, Spain
dInstitute of Molecular Genetics AS CR, Flemingovo n. 2, 16637 Prague, Czech Republic, current address: Dep. Biochemistry, UT Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, Texas 75390-8816
eBiological Centre IPMB AS CR, Branisovska 31, 370 05 Ceske Budejovice, Czech Republic
Photosystem II (PS II) is located in the thylakoid membrane of higher plants, algae and cyanobacteria. Its function to capture sunlight is realized by two antenna proteins CP47 and CP43. They transfer the excitation energy to the photochemical reaction center with primary electron donor P680, which is formed by chlorophyll α molecules [1-2]. This large multisubunits protein-pigment complex reduces bound of quinine during oxidizing water with releasing of oxygen molecule. PS II consists of four membrane-internal subunits (D1, D2, CP43, CP47), several smaller internal membrane (including PsbE and PsbF, constituting cyt b-559) and three external subunits (PsbQ, PsbP, PsbO in green algae and higher plants) [3].
Experimental
part:
Crystallization of macromolecule complex such as PS II is influenced by many parameters, from which is the most important the protein itself, as its purity, homogeneity and some other properties, but mainly its disposition to form crystals. This tendency to produce for diffraction measurement suitable crystals may be optimized combination of using different crystallization techniques and other physicochemical parameters (precipitants, additives, pH, etc.) influencing crystallization [4-5].
Nowadays counter-diffusion method becomes very popular like forth out of common vapor diffusion techniques: sitting and hanging drops, microbatch under oil, etc. Capillaries of different length and diameters performed in the Granada Crystallization Boxes (GCB-Domino, Triana Sci&Tech, Granada, Spain) are a good example of counter-diffusion technique. In fact, the protein sample in solution contacts precipitant directly. Capillaries (Fig.1) filled by protein {1} are placed into the different precipitant solutions {2} through 0.1% agarose {3} to fix them. Gradient of supersaturation appears along the length of the capillary due to diffusion of solutions against each other. Optimal condition for producing nice single crystals suitable for X-ray diffraction measurement can arise in some parts of capillary.
The sample with dimeric core complex of PS II (OEC PSII) was obtained form Pisum Sativum. Aim of our project is based on using advanced counter-diffusion and standard vapor-diffusion methods, to observe capability of individual precipitants to have an influence on crystals growth. Following results described by Kuta Smatanova et al. [6] many other chemical components such as detergents for membrane protein (β-DM, C12E8), buffers with different pH (MES, HEPES, Tris, KH2PO4), cryoprotectants (PEG with several molecular weight, Glycerol, MPD) were tested again. We have been also investigating the influence of whole spectrum of salt additives from Hampton Research screening test (Ba, Mg, Ca, Mn, Cd, Cu, Co, Cs, Zn, Y, Ni, Sr) (Hampton Research, CA, USA) due to find suitable condition to produce nice single crystals. Crystals with hexagonal shape and needles were obtained from different conditions.
Computational
part:
Excitonic interaction between chlorophyll molecules is a general phenomenon in photosynthetic reaction centers. Strong interaction between closely located molecules of pigments leads to a situation where these molecules lose their individual identity and their spectral properties change significantly. The optical properties of chlorophyll-pheophytin complex are determined by the strong interactions between the pigments which are nearly at Van der Waals’ distance to each other.
Exciton interactions between pigments of photosystem II reaction center (PSII RC) from higher plants upon oxidation or reduction of primary acceptor pheophytin (D1-Pheo) have been studied by us using experimental and molecular modeling techniques [7, 8]. For characterization of the spectral properties (absorption and CD spectra) of multimer protein-pigment system, Exciton Limit (Matrix Method) [9] was applied on crystal structure of PSII RC (accession code in protein databank 1S5L [10]). This method consists of two parts, in the first part, one finds the eigenvalues and eigenvectors of the matrix, and the off-diagonal elements are interaction energies between the molecules (pigments) and the diagonal terms represent individual transition energies of pigments. Interaction energies were calculated by two ways, the point dipole and improved point monopole method. The point-dipole method [11] evaluates interaction energy between the transition dipole moments of pigments. The point-monopole method [12] describes the interaction energy between transition monopoles distributed on the pigments. In order to obtain realistic light-adapted absorption spectra of PSII RC we modified site energies (individual transition energies of pigments) by so-called electrochromic shifts as interaction energies between atomic partial charges of D1-Pheo and differential dipole moments of the excited and ground states of PSII RC core pigments [13]. In the second part of calculation, the eigenvectors of this matrix were applied along with the molecular electric transition dipoles to calculate absorption intensity for each of the matrix eigenvalues.
The results of these calculations are a representation of aggregate optical absorption and CD spectra in the form of line spectra. Each line was superimposed by a smooth gaussians function to give the appearance of actual experimental spectra.
Acknowledgements:
This
work is supported by grants NSM6007665808 and LC06010 of the Ministry of Education
of Czech Republic and Institutional research concept AVOZ60870520 of Academy of
Science of Czech Republic.
References:
[1] J.
Biesiadka, B. Loll, J. Kern, K.-D. Irrgang, A. Zouni, Phys. Chem. Chem.
Phys, 6 (2004) 4733-4736.
[2] R. Anati, N.
Adir, Photosyntesis Research, 64 (2000) 167-177.
[3] J. Kern, B.
Loll, C Luneberg, D.DiFiore, J. Biesiadka, K.-D. Irrgang, A. Zouni, Biochimica
et Biophysica Acta, 1706 (2005) 147-157.
[4] I. Kuta Smatanova, J.A. Gavira, P. Rezacova, F. Vacha, J.M. Garcia-Ruiz, Acta Cryst. A61 (2005) C147.
[5] K.N. Ferreira, T.M. Iverson, K. Maghlaoui, J. Barber, S. Iwata, Science, 303 (2004) 1831-1838
[6] I. Kutá Smatanová, J. A. Gavira, P.
Řezáčová, F. Vácha, and J. M. García-Ruiz, accepted for publication in Photosynthesis
Research (2007).
[7] F. Vácha, M. Durchan and P. Šiffel, Biochimica
et Biophysica Acta, 1554 (2002) 147.
[8] F. Vácha, J.
Pšenčík, M. Kutý, M. Durchan and P. Šiffel, Photosynthesis Research, 84 (2005) 297.
[9] P. M.
Bayley, Prog. Biophys. 27 (1973) 3-76.
[10] K.N. Ferreira, T.M. Iverson, K.
Maghlaoui, J. Barber, S. Iwata, ![]() Science,
Science,
![]() 303
(
303
(![]() 2004)
1831-1838.
2004)
1831-1838.
[11] V.I.
Prokhorenko, D.B. Steensgaard, A.R. Holzwarth: Biophysical Journal, 85
(2003) 3173-3186.
[12] J. C.
Chang, Chem. Phys., 67 (1977) 3901-3909.
[13] G. Raszewski, W. Seanger, T. Renger: Biophysical
Journal, 88 (2005) 986-998.
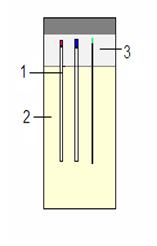
Figure 1. The Granada Crystallization Box
with capillaries of different diameters (1 –
capillary, 2 – precipitant, 3 – agarose)



Figure 2.