Crystallographic studies of intermolecular interactions of Fc-fragment of immunoglobulin
P. Kolenko1,2,
J. Dohnálek1, J. Dušková1, J. Hašek1, T. Skálová1,
1Institute of Macromolecular Chemistry, AS CR, v.v.i., Heyrovskeho nam.2, 162 00, Prague 6
2Dept.of Solid State Physics, FJFI, CTU, Trojanova 13, 120 00, Prague 2
kolenko@imc.cas.cz
Keywords: antibody, glycosylation, conformation changes, ligand.
IgG antibodies are one of the key mediators of immune system response to some viral infections. They are also used in medicine for many different purposes, such as strengthening of immune system, detection of primary tumors and microscopic metastasis using scintigraphy, etc.
Antibodies are composed of three globules (fragments): two Fab-fragments (Fragment-antigen binding), Fc-fragment (Fragment-crystallizable). Fab-fragments are responsible for antigen detection, Fc-fragment (tertiary structure shown in Fig. 1) plays an important role in immune system activation. In our structure solution [1], the Fc-fragment of mouse monoclonal IgG2b against carbonic anhydrase MN CA IX was cleaved from intact antibody with papain.
The Fc-fragment is functional as a dimer. A monomer is formed by two domains, named Cγ2 and Cγ3, joined by a linker composed of four amino acids. Two Cγ3 domains form a compact non-covalent dimer. The Cγ2 domains are linked by hydrogen bonds between oligosaccharides covalently attached to asparagines, disulfide bridges between cysteins in the region linking these domains to the Fab fragments were found only in a few structures in the PDB database [2]. In our case [1], the area of disulfide bridges is disordered (flexible) and without a possibility of clear structure interpretation.
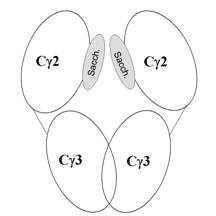
Figure 1. Diagram of tertiary structure of the Fc-fragment
of an antibody.
The PDB database [2] contains thirty records with structures of Fc-fragment. They can be divided into four groups: Fc-fragments cleaved from antibodies (by papain, pepsin, etc.), intact antibodies, Fc-fragment in complex with a ligand attached via the Cγ2-Cγ2 interface, Fc-fragment in complex with a ligand attached via the Cγ2-Cγ3 interface. An extensive analysis of structure and interaction of oligosaccharide chains was performed and the results summarized.
The project is supported by the Ministery of Education, Youth and Sports of the Czech Republic (project no. 1K05008) and by the Commission of the European Communities (project no. LSHG-CT-2006-031220).
1. P. Kolenko, J. Dohnálek, R. Šťouračová, T. Skálová, G. Tiščenko, J. Dušková, J. Hašek, Materials Structure, 12, (2005), 146.
2. H.M.
Berman, J. Westbrook, Z. Feng, G. Gilliland, T.N. Bhat, H. Weissig, I.N.
Shindyalov, P.E. Bourne. Nucleic Acids
Research, 28, (2000), 235.