Keywords: cytochrome, electron transfer,
crystallization, diffraction
Introduction
The cytochromes are ubiquitous proteins present
in all living organisms and involved in a variety of intracellular processes
that are essential for life. Most notable is their participation in electron
transfer reactions, usually as components of a complex reaction pathway,
necessary for the production of energy either through oxidation of metabolites
or via photosynthesis. Cytochromes are members of a larger class of proteins,
known as hemoproteins. The hemoproteins derive their name from the presence of
one or more iron porphyrin prosthetic groups (called as hemes). Besides
cellular bioenergetics, the heme is also involved in ligand binding reactions
necessary for oxygen transport [1].
Compared with other biologically active
molecules, cytochromes are some of the simplest bioinorganic compounds considering
of molecular weight and structure. The active center of cytochromes is the heme
group [2]. It consists of a porphyrin ring chelated to an iron atom. The
porphyrin ring is a macrocyclic pyrrole system with conjugated double bonds.
These compounds undergo chemical oxidation and reduction, cycling between
ferrous (Fe2+) and ferric (Fe3+) forms, in contrast to
the hemoglobin, where the iron is normally in the ferrous (Fe2+)
state.
Our
cytochrome is characterised by alpha-peak wavelength of 553 and a molecular
mass of 25 kDa. The absorption spectrum (performed by UV-visible absorption
spectroscopy [3]) presents distinct split alfa band, and a very low alfa
to beta ratio. The protein consist of two heme
molecules in single polypeptide chain with classical Cys–X–Y–Cys–His heme
binding sites. The fifth heme iron ligand is always provided by a histidine
residue [2]. Cytochrome is located probably in bacterial periplasmic space of Thiocapsa roseopersicina cell wall. The established
properties of this hemoprotein indicate that it belongs to the c4
family [3] of diheme cytochromes. It is the first cytochrome of this class that
comes from an anaerobic organism. Due to its important function, it is of
essential interest to study structural features of cytochromes using X-ray
crystallography.
Materials and crystallization methods
Cytochrome
c4 (cyt c4) from the purple photosynthetic bacterium Thiocapsa
roseopersicina was isolated and purified according to [3]. This bacterium
has four different hydrogenases and three different cytochromes. The cyt c4
has been studying by crystallographic, proteolytic [4] and spectroscopic
methods. Cyt c4 was crystallized using standard crystallization
methods based on vapor diffusion (hanging and sitting drops [5]) and advanced
crystallization method based on the counter-diffusion (crystallization in
capillaries [6]).
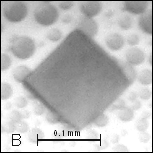
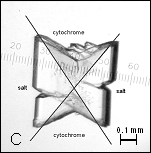
Figure
1: A, B –
Pseudocrystals of cyt c4 and C – crystal of cyt c4
constructed together with AS.
Initial crystallization trials with ammonium sulfate (AS) yielded pseudocrystals as red thin plate [Figure 1] with components from 0.1 M sodium chloride and 0.1 M citric acid pH 6.0 in the reservoir solution. Ranging pH value higher than 7.5 the phase separation of protein appeared.
Crystallization trials were performed at 20 °C. After
fine-tuning crystallization conditions, the most suitable concentration of
protein (10–15 mg/ml) and the percentage of precipitation agent were found. The
first suitable crystal growth was observed at pH 6.0 [Figure 2] using the
addition of metal ions – Cu2+, Cd2+, Co2+, Ba2+
(from Hampton Research Additive Screen HR2–428). Cyt c4 crystals were grown in
capillaries when the precipitating system contacts the protein solution because
a wave of supersaturation was triggered.
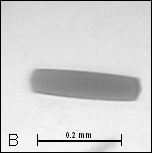
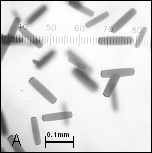
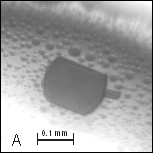
Figure
2: A, B –
Crystals of cyt c4
Diffraction
measurement
The monocrystals
of cyt c4 were tested at the home source diffractometer at LEC (
Colored crossbred plates of holoprotein crystals with
dimensions of approximately 230 x 40 x 20 μm grew within 3–4 days under
several conditions. Protein crystals grown in capillaries were measured
directly at synchrotron DESY (
References
1. G. Moore, F. Pettigrew:
Cytochromes c,
2. T. Yamanaka: The Biochemistry Of
Bacterial Cytochromes,
3. Cs. Bagyinka, R. M. M. Branca:
unpublished data (2005)
4. J. Carey: Methods Enzymol., Vol.
328, 449 (2000)
5. T. M. Bergfors: Protein Crystallization.
Techniques, Strategies and Tips.
6. F. J. López-Jaramillo, J. M.
García-Ruiz, J. A. Gavira, F. Otálora: J. Appl. Cryst. 34, 365-370 (2001)
Acknowledgements
This work is supported by
grants of the Ministry of Education of the
the Czech Republic (MSM6007665808, LC06010) and by the Academy of Sciences of
the Czech Republic (Institutional research concept AVOZ60870520).