STRUCTURAL STUDIES OF GALECTIN-4 N-TERMINAL DOMAIN IN COMPLEX WITH LACTOSE
Veronika Krejčiříková1, Jiří
Brynda1, Milan Fabry1, Gabriela Jeníková2,
Vladimíra Marková2, Petr Malý2
1Department of Recombinant Expression and Structural Biology, Institute of Molecular genetics, Flemingovo nám. 2, 166 37 Praha 6
2Department of Molecular Glycobiology, Institute of Molecular genetics, Vídeňská 1083, 142 20 Praha 4
Galectins are an evolutionarily conserved family of 15 different lectins found in various combinations in virtually every type of animal cell. One of the primary galectins expressed in intestinal epithelium is galectin-4, a tandem-repeat galectin with two carbohydrate-recognition domains in a single polypeptide chain having affinity for ß-galactosides as the lactose [1].
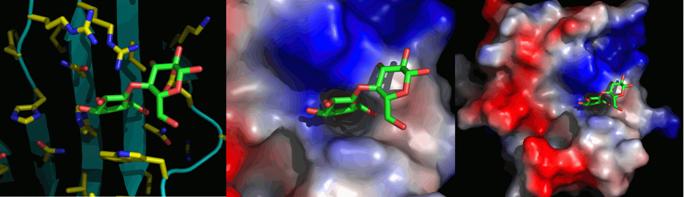
We reported here the x-ray structure of mouse galectin-4 N-terminal domain (CRD1) in complex with lactose (Figure1) at resolution 2.1 Å. Comparison with other galectins, mode of ligand binding and fluorescence of interacting Tryptophan will be discussed.
a)
b)
c)
Figure
1
a) Detail
view of interacting lactose (carbons coloured green) in binding site.
b) The
same as a), solvent accessible surface of galectin is coloured by electrostatic
potential (negative red, positive blue).
c) General
view of the complex CRD1-lactose.
1. M.A.Wooters, S.L.Ropp, A K.Erickson, Biochimie., 87 (2005) 143-149