COMPUTATIONAL ANALYSIS OF ENZYME ACTIVE SITE ROUTES ANATOMY
Petřek Martina, Otyepka Michalb, Banáš Pavelb and Damborský Jiřía
a National Centre for Biomolecular Research, Masaryk University, Kotlářská 2, 611 37 Brno, Czech Republic
b Department of Physical Chemistry, Palacký University Olomouc, tř. Svobody 26, 771 46 Olomouc, Czech Republic
Enzymes are catalysts of biological systems that determine the patterns of chemical transformations. The enzymatic reaction is complex, consisting of substrate binding, one or more chemical reactions and product release. It is necessary to consider all these steps in study of enzymatic activity. The rate-limiting step can be substrate binding or product release in some instances. The molecular dynamics simulation is effective tool to study flexibility of biomolecules. The goal of this work was to develop a simple, quick and flexible technique to monitor radius of a tunnel leading to the active site during molecular dynamic simulation. The presented algorithm utilizes alpha shape theory in combination with grid search algorithm [1].
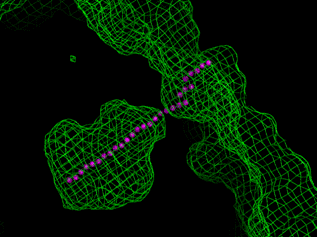
The haloalkane dehalogenases (EC 3.8.1.5) are microbial enzymes cleaving a carbon-halogen bond in a broad range of halogenated compounds [2]. These enzymes were used for the case study. The X-ray structures of three haloalkane dehalogenases are known: LinB from Sphingomonas paucimobilis UT26 [3], DhlA from Xanthobacter autotrophicus GJ10 [4] and DhaA from Rhodococcus sp. NCIMB13064 [5]. The active site is buried between the main domain (the α/β hydrolase fold domain) and the cap domain. The several possible access routes were denoted as main tunnel, upper tunnel and slot. The enzymes LinB, DhaA and DhlA differ in number of routes as well as in radius of each tunnel leading to the active site. LinB has the most open active site accessible through all three tunnels, DhaA has accessible upper tunnel and slot and DhlA has only one access via main tunnel [6]. Number and size of entrance tunnels seems to correlatewith the rate limiting step. The rate-limiting step of DhlA and DhaA is a product release, while the chemical reaction is the rate-limiting step of LinB [7]. The amino acid Leu177 of LinB localized in a gorge of upper tunnel was mutated to study the influence of upper tunnel radius upon enzyme activity. The substitution in analogous amino acid of DhaA, Cys176, was obtained by direct evolution method. Cys176Tyr mutation increased enzyme activity with 1,2,3-trichloropropane 3-times [8]. The developed program was used to determine the preferred route to the active site (Fig 1.) in the molecular dynamic simulations of wild type enzymes DhaA, DhlA, LinB and Cys176Tyr mutant of DhaA in complexes with 1,2,3-trichloropropane. The free enzymes differ in radii of access route gorge and also in mean path cost (Table 1). DhlA has the narrowest and expensive active site route while DhaA and LinB tunnels have higher radii and are cheap. Cys176Tyr mutation (DhaA) causes change in preferred (low cost) route form upper tunnel (WT-DhaA) to slot (Cys176Tyr-DhaA).
Table 1 Mean
radii (Å) of active site access routes and mean route costs. The
normalized cost function is defined as![]() , where rmax(x) is the maximal
radius of a hypothetical ball that can be inserted into the node x just
touching the protein surface. The small constant ε is here only for
technical purposes to get rid of a singularity of the function in points where rmax(x)
equals zero, N is number of path nodes.
, where rmax(x) is the maximal
radius of a hypothetical ball that can be inserted into the node x just
touching the protein surface. The small constant ε is here only for
technical purposes to get rid of a singularity of the function in points where rmax(x)
equals zero, N is number of path nodes.
|
enzyme |
radius |
cost |
|
DhlA |
0.3 ± 0.1 |
2.1 ± 2.0 |
|
LinB |
0.7 ± 0.3 |
1.6 ± 1.2 |
|
DhaA |
0.9 ± 0.2 |
0.5 ± 0.1 |
|
Figure 1 Active site of LinB enzyme represented by solvent accessible surface (wire model) and the easiest way from the active site (magenta points), calculated at 60 x 60 x 60 grid |
|
References:
1. Dijkstra, E.W., A note on two problems in connection with graphs. Numer. Math., 1959. 1: p. 83-89.
2. Damborsky, J., et al., Structure-specificity relationships for haloalkane dehalogenases. Environmental Toxicology and Chemistry, 2001. 20(12): p. 2681-2689.
3. Nagata, Y., et al., Purification and characterization of haloalkane dehalogenase of a new substrate class from a g-hexachlorocyclohexane-degrading bacterium, Sphingomonas paucimobilis UT26. Applied and Environmental Microbiology, 1997. 63: p. 3707-3710.
4. Keuning, S., D.B. Janssen, and B. Witholt, Purification and characterization of hydrolytic haloalkane dehalogenase from Xanthobacter autotrophicus GJ10. Journal of Bacteriology, 1985. 163: p. 635-639.
5. Kulakova, A.N., M.J. Larkin, and L.A. Kulakov, The plasmid-located haloalkane dehalogenase gene from Rhodococcus rhodochrous NCIMB 13064. Microbiology, 1997. 143: p. 109-115.
6. Otyepka, M. and J. Damborsky, Functionally relevant motions of haloalkane dehalogenases occur in the specificity-modulating cap domains. Protein Science, 2002. 11(5): p. 1206-1217.
7. Chaloupkova, R., et al., Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by engineering of its entrance tunnel. Journal of Biological Chemistry, 2003. 278(52): p. 52622-52628.
8. Bosma, T., et al., Biodegradation of 1,2,3-trichloropropane through directed evolution and heterologous expression of a haloalkane dehalogenase gene. Applied and Environmental Microbiology, 2002. 68(7): p. 3582-3587.