Structural Studies of Carbonic Anhydrase IX
Pavel Mader, Renata
Štouračová, Jiří Brynda, Milan Fábry, Magdalena Hořejší,
Vlastimil Král, Juraj Sedláček
Department of
Recombinant Expression and Structural Biology, Institute of Molecular Genetics,
Flemingovo nám. 2, CZ - 166 37 Praha 6, mader@img.cas.cz
Carbonic anhydrase IX (CA IX) belongs to a
family of zinc metalloenzymes that catalyze the reversible hydration of carbon
dioxide: CO2 + H2O → H+ + HCO3-. These enzymes play a role in pH regulation, CO2 and HCO3-
transport, bone resorption, production of biological fluids, ureagenesis,
gluconeogenesis, and lipogenesis [[1]]. CA IX is a unique member of the
CA protein family. In contrast to the other isozymes, it has been implicated to play a role in
regulation of cell proliferation, adhesion, and malignant cell invasion [[2], [3]]. This integral membrane protein was shown to
function as a cell
adhesion molecule with the binding site localized in the extracellular
proteoglycan-like (PG) domain; the same site is recognized by the monoclonal
antibody M75 thus abrogating cell adhesion in in vitro test [2]. Interestingly, CA IX is
overexpressed in human epithelial tumours derived from tissues that normally do
not express this isozyme, including carcinomas of cervix, lung, kidney, and
breast [4]. In contrast, tumours originating from CA IX-positive tissues, such
as stomach, tend to have lowered expression of CA IX [[4]]. CA IX is also overexpressed in
von Hippel-Lindau (VHL)-defective tumors and under hypoxic conditions [[5]]. There are several reasons to consider CA IX
as a suitable target molecule for cancer therapy: (i) it is expressed
ectopically in various commonly occurring carcinomas, which are rather
resistant to conventional therapy; (ii) the antigen is exposed on the cell
surface; (iii) normal expression of CA IX is restricted to the luminal
epithelia of the alimentary tract, with limited accessibility to immune cells,
antibodies and many drugs [[6]].
The aim of our research is to help elucidate the molecular basis of CA IX involvement in cell proliferation by solving the structure of recombinant forms of extracellular domain of CA IX. Further, we plan to solve structure of CA IX complexes with specific sulfonamide inhibitors, and recombinant scFv fragment derived from monoclonal antibody M75, and finally to identify the putative CA IX binding partner.
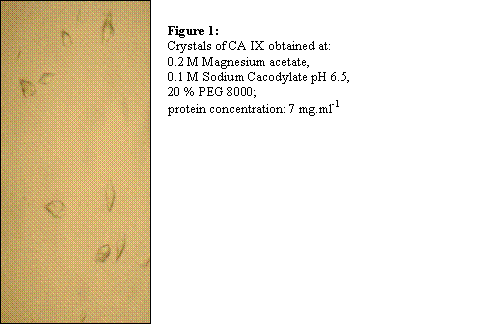
To obtain recombinant extracellular CA IX domain, various expression systems were tested, however only Drosophila Schneider 2 Cells yielded sufficient amount (60 mg.l-1) of native protein in serum free medium. The protein was purified in 3 chromatographic steps: affinity chromatography on p-aminobenzene sulfonamide agarose, anion exchange chromatography, and gel filtration. The first crystals of CA IX have been obtained using vapour diffusion hanging drop method [Figure 1].
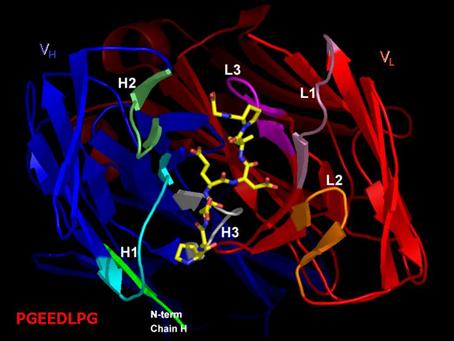
 Structure of M75 Fab fragment in complex with its epitope
peptide [Figure 2] derived from PG domain of CA IX, which will also be
presented, should be helpful in rational drug design of compounds suitable for
use in human oncology.
Structure of M75 Fab fragment in complex with its epitope
peptide [Figure 2] derived from PG domain of CA IX, which will also be
presented, should be helpful in rational drug design of compounds suitable for
use in human oncology.