Membrane Transport without Receptors?
THE ROLE OF cyclosporines and silymarines STRUCTURES FOR THEIR INTERACTIONS with lipidS of hepatocyte plasma membrane.
Jiří Šebestian,1,2, Štěpánka B. Šebestianová,3,
Vladimíra Moulisová,1,2, and Alexandr Jegorov,4
1 Institute of Physical
Biology, Univ. South Bohemia, Nový Zámek 136, CZ-373 33 Nové Hrady, CZECH
REPUBLIC
2 Laboratory of Biomembranes,
Fac. Biol., Univ. South Bohemia, Branišovská 31, CZ-370 05 České Budějovice,
CZECH REPUBLIC
3 Dept. Genetics, Fac. Biol.,
Univ. South Bohemia, Branišovská 31, CZ-370 05 České Budějovice, CZECH REPUBLIC
4 IVAX CR, Research Unit,
Branišovská 31, CZ-370 05 České Budějovice, Czech
Republic
Address for correspondence:
E-mail:
sebest@jcu.cz
This work was supported by grants: GACR 204/98/P129, MSMT CEZ:J06/98:123100001 and GACR 521/99/D098.
INTRODUCTION:
Cyclosporines (Cs) are non-ribosomatically synthesized toxic cyclic
undekapeptides that include some non-coded aminoacids. Well known and the most
common of them is cyclosporine A (CsA) which is widely used in medicine as
a powerful immunosuppressant in organ transplantations and for suppression of multidrug resistance in tumor chemotherapy. The
cyclopeptidic structure features have some other important natural toxins (i.e.
alga toxin microcystine, mushroom toxins amanitin and phalloidin etc.).
Flavanolignans (called silymarines) from Milk Thistle (Silybum marianum) are known to help against hepatotoxic effects of
cyclic peptides. Mechanisms of actions in the liver cell are well known for
both cyclosporines and silymarines. But much less is known about their
transport through the cell plasma membrane. Because of their hydrophobic nature
they are believed (at least partly) to enter the cell by passive diffusion
through the lipidic part of hepatocyte plasma membrane.
We investigated the interaction of these compounds with different models
of hepatocyte plasma membranes (from different phospholipid vesicles through
plasma membrane vesicles up to hepatocytes). The interactions were monitored by
changes in membrane lipid fluidity after cyclosporine or silymarine addition.
The membrane fluidity was observed by measuring of the steady-state
fluorescence anisotropy of diphenylhexatriene (DPH) and its polar derivative,
TMA-DPH.
RESULTS AND DISCUSSION:
We found that for interactions of cyclosporines with lipids is the most
important the side chain of the second aminoacid. The membrane fluidity
increases in the raw: CsC << CsA < CsD <
CsG, where the second aminoacid in CsC is threonin, in CsA: a-aminobutyric acid, in CsD: valin
and in CsG: norvalin. The same membrane fluidity as in case of CsA was found
for interactions of cyclosporines with changes in other than second aminoacid;
including CsH, which 3D structure completely differs from other cyclosporines
(position 11 D-N-methylvalin instead of L-MeVal). The role of the second amino
acid is supported by the fact that this side chain is in 3D structure of
cyclosporines the most exposed residue to the space above the plane of
cyclopeptide ring. On the other hand the change in amino acid number 1 which is
the most exposed residue under the cyclopeptide chain, has no effect (i.e.
fluidity measured for AcetylCsA was the same as for CsA). These results and the
same patterns were found for each type of vesicles and for both probes. The
changes were in order of 10-3 anisotropy units.
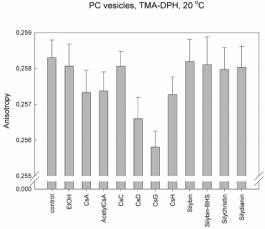
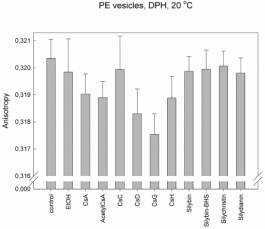
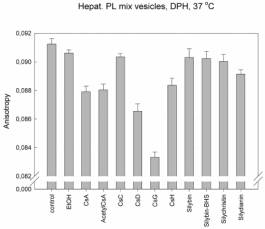
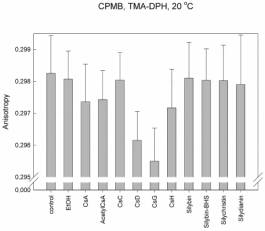
Explanation
to figures:
The pattern of anisotropy values was the same at 37 °C as in case of 20
°C (compare Fig. 3.) but the standard deviations were much higher. Thus we
decided to measure at 20 °C.
TMA-DPH: Amphiphilic fluorescence probe
1-[4-(trimethylamino)phenyl]-6-phenyl-hexa-1,3,5-triene.
DPH:
Hydrophobic fluorescence probe 1,6-diphenyl-hexa-1,3,5-triene
PC:
Vesicles prepared from pure phosphatidylcholine – the main phospholipid of
hepatocyte membrane that dominates in the inner half-layer of hepatocyte
membrane.
PE:
Vesicles prepared from pure phosphatidylethanolamine– the second main
phospholipid of hepatocyte membrane that dominates in the outer half-layer of
hepatocyte membrane.
Hepat. PL:
Vesicles prepared from the mixture of phospholipids isolated by
chlorofom-methanol extraction of hepatocyte plasma membranes
CPMB:
Vesicles prepared from cytoplasmic membranes of hepatocytes = with proteins
But
different results and patterns were found in case of hepatocytes. Changes in
membrane fluidity were found only in case of CsG but in the order of 10-2
anisotropy units!
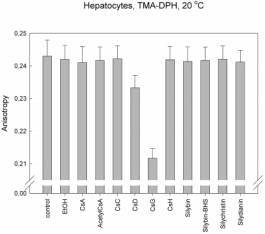
No changes
were found in case of silymarines.
CONCLUSIONS:
We
concluded that:
1) The
most important for hydrophobic interaction of cyclosporines is the second amino
acid residue.
2) The
intensity of the interaction increase with hydrophobicity of the second amino
acid residue.
3)
Hydrophobic interactions of cyclosporines in vivo can play a role only
in case of CsG.
Materials and
methods:
DPH and TMA-DPH were purchased from Molecular Probes (USA). All the
other chemicals used were of analytical grade from Sigma-Aldrich (Czech
Republic).
1. Animals
Two months old male Wistar rats weighing 200-250 g were used for
hepatocyte isolation, liver cytoplasmic membrane preparation and liver
phospholipid isolation. Animals were kept in standard laboratory conditions
with free access to water and standard pellet laboratory diet (KrmiMo Mohelski,
Brno, Czech Republic). All procedures with animals were approved by the Ethics
Commettee, Ministery of Education, Czech Republic.
2. Hepatocytes preparation
Rat hepatocytes were isolated by modified two-step collagenase perfusion
(Moldeus et al., 1978). The hepatocytes were collected in PBS, filtered on
gauze and washed three times by centrifugation (50 g). Cells were resuspended
in William’s medium E and washed once more by centrifugation (50 g). Cells were
counted using trypan blue exclusion test and viability was typically 90 %.
Freshly prepared hepatocytes were resuspended in William’s E medium
supplemented by 1 % bovine serum to the final concentration 6´105 cells/ml.
3. Cytoplasmic membrane vesicles isolation
Cytoplasmic membranes from hepatocytes were isolated by differential
centrifugation and isopycnic centrifugation using discontinual sucrose gradient
according to Scott et al. (1993).
4. Phospholipid isolation from hepatocytes
Phospholipids were isolated by classical chloroform/methanol extraction
with modification according to Cartwright (1993) from isolated hepatocyte
plasma membranes. Isolated phospholipids were stored dissolved in chloroform
under nitrogen in –70 °C before using.
5. Phospholipid vesicles preparations
Monolayer phospholipid vesicles were prepared from commercial
phosphatidylcholin from egg yolk and phosphatidyl ethanolamine from sheep brain
(Sigma-Aldrich, Prague, Czech republic) or from isolated hepatocyte plasma
membrane phospholipids by very gentle sonication for 1 minute in 4 °C under nitrogen atmosphere.
6. Labelling of hepatocytes, plasma membrane vesicles and phospholipid
vesicles
DPH in final concentration 5´10-7 M was directly added to the stirred sample and let to
incorporate for 30 minutes at 37 °C. Incorporation of TMA-DPH was very quick (less than 1 minute) thus
TMA-DPH was added directly to the sample in the fluorescence cuvette to the
final concentration 5´10-7 M. Final
volume in the cuvette was 2 ml. Final concentrations of ingredients were:
hepatocytes 3´105 cells/ml (=75 mg of protein/ml), membrane vesicles 75 mg of protein/ml, phospholipid vesicles 0.3 mg/ml,
investigated compound (silymarine or cyclosporine) 10 mM. Fluorescence anisotropy was measured as soon as
possible after the investigated compoud addition (30 seconds) and each 5 minute
after that up to 30 minutes. Fluorescence probes and investigated compound were
dissolved before addition in spectroscopic pure ethanol and final concentration
of ethanol was 0.5 % (v/v).
7. Fluorescence anisotropy measurement
Steady-state anisotropy measurements were performed with a SLM 4800S
fluorometer (SLM Instruments, Urbana, IL), equipped with the standard
polarization accessory. The excitation wavelength was set to 360 nm.
Fluorescence was detected through a cut-off emission filter (Schott, 50%
transmittance at 405 nm). And the emission monochromator was set to 430 nm. The
correction for the fluorescence intensity of non-labelled hepatocytes (usually
10 % intensity of the TMA-DPH labelled cells) was calculated according to Kuhry
et al., (1985). The background fluorescence of non-labelled vesicles of all
types did not exceed 1% of the experimental values. The steady-state anisotropy
was calculated according to Lakowitz (1983). The steady-state anisotropy values
presented in this work correspond to the average of at least three
determinations performed with the independent vesicle preparations.
8. Other methods.
Protein concentrations were determined by the Lowry et al. (1951) assay
in the presence of 0.07 % (w/v) sodium deoxycholate in alkali pH using BSA as
the standard.
LITERATURE:
Moldeus
et al. (1978) Methods Enzymol. 52:
62-71
Scott et al. (1993) Methods Mol. Biol. 19: 59-69
Cartwright (1993) Methods Mol. Biol. 19: 153-161
Kuhry et al. (1985) Biochim. Biophys. Acta 845: 60-67
Lakowitz (1983) Principles of Fluorescence Spectroscopy, Plenum Press,
New York
Lowry et al. (1951) J. Biol. Chem. 193: 263-272