THEORETICAL STUDY OF 31P CHEMICAL SHIELDING TENSORS IN B-DNA
Jana Přecechtělová, Markéta L. Munzarová and Vladimír Sklenář
National Centre for Biomolecular
Research, Faculty of Science, Masaryk University, Kotlářská 2, CZ-611 37 Brno,
Czech Republic
Isotropic 31P chemical shielding (CSI) and chemical shielding anisotropy (CSA), the quantities derived from 31P chemical shielding tensors, provide valuable information on the sugar-phosphate backbone conformation in nucleic acids [1]. The backbone conformation between two successive phosphorus atoms is defined by six torsional angles. As evidenced by previous theoretical studies [2, 3, 4], the two PO ester torsional angles, a (O3'–P–O5'–C5') and z (C3'–O3'–P–O5') (Fig. 1), are the ones playing the prior role in determining 31P chemical shielding. In addition, the effects of the OPO bond angle and CO torsional angles, e.g., e (C4'–C3'–O3'–P) (Fig. 1), seem to be essential [2, 5].
In our work we apply density functional theory (DFT) to assess the influences of the a, z, and e torsional angles, as well as the OPO bond angle on 31P chemical shielding. In the geometry optimizations of a model compound (Fig. 1) only the torsional angle studied has been varied within its experimental range and all other backbone torsions have been kept frozen. In order to conduct NMR parameter calculations the deMon-NMR code [6] has been used.
Our results reveal that CSI as well
as CSA increase when either a or z goes
up from 270° to 330° for the former or from 240° to 300°
for the latter. The range of CSI as a function of both torsional angles is of
an order of only a few ppm. On the contrary,
CSA changes within 30 ppm in the case
a and
within 10 ppm in the case of z. The larger ranges of CSI/CSA obtained for a (compared with z) indicate higher
sensitivity of the 31P chemical shielding to the a torsional angle. The
plots of CSI/CSA against a (z ) obtained for various values of zÎá240°,300°ñ
(aÎá270°,330°ñ) show that the trends of CSI/CSA are shifted to higher
values when z (a) increases from 240° to 300°
(from 270° to 330°).
Interestingly, changes in CSI
introduced by the e torsional angle are comparable with those introduced by a and z. However, e leaves CSA almost
unaffected. Furthermore, we have uncovered that the trend in the dependence of
CSI on a correlates
with changes in the OPO bond angle caused by changing the a torsional angle. Last but not least
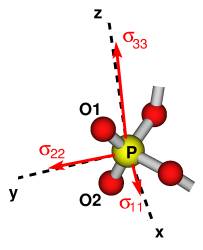
our calculations show that for any choice of alpha, zeta, and epsilon, the
directions of the principal components of the 31P chemical shielding
tensors almost coincide with the axes of the Cartesian coordinate system
defined so that the y axis bisects the O1–P–O2
angle, the z axis lies in the O1–P–O2 plane and is perpendicular to y,
and the x axis is orthogonal to both y and z (Fig.
2).
Acknowledgment:
This work was supported by grant
LN00A016 from the Ministry of Education, Youth and Sports.
1. D. G. Gorenstein, Chem. Rev., 94 (1994) 1315–1338.
2. D. G. Gorenstein, D. Kar, Biochem. Biophys. Res.
Commun., 65 (1975) 1073–1080.
3. F. R. Prado, C. Giessner-Prettre, B. Pullman, J-P. Daudey, J.
Am. Chem. Soc., 101 (1979) 1737–1742.
4. C. Giessner-Prettre, B. Pullman, F. R. Prado, D. M. Cheng,
V. Iuorno, P. O. P. Ts'o, Bioplomers, 23 (1984) 377–388.
5. D. G. Gorenstein, J. Am. Chem. Soc., 97 (1975) 898–900.
6. V. G. Malkin, O. L. Malkina, L. A. Eriksson, D. R. Salahub:
Modern Density Functional Theory: A Tool for Chemistry, J. M. Seminario,
P. Politzer, Eds., Elsevier: Amsterdam, 1995.
Figure 1:
The model used for studying the influences of the a, z,
and e torsional
angles, as well as the OPO bond angle on 31P chemical shielding
tensors.
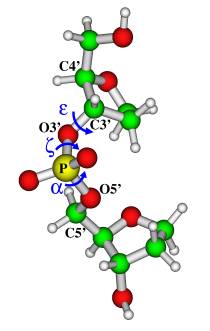
Figure 2: The orientations of the principal components of the 31P
chemical shielding tensor with respect to the axes of the Cartesian coordinate
system.