Structural measurements on
membrane PsbH protein
Zbyněk
Halbhuber+,
Norbert Müller*, Ivana
Kutá Smatanová+,$, Julie
Wolfová+,
Dalibor Štys+,$,#
+ Institute of Physical
Biology, University of South Bohemia, Zámek
136, 373 33 Nové Hrady, Czech Republic
* Johannes Kepler Universität, Altenbergerstrasse 69, Linz, Austria
$ Institute of Landscape
Ecology, Academy of Sciences of the Czech Republic, Zámek 136, 373 33 Nové Hrady, Czech Republic
# Institute of Microbiology,
Academy of Sciences of the Czech Republic, Opatovický mlýn, 37901 Třeboň, Czech Republic
Introduction
The psbH protein is one of key components for assembly of Photosystem II [1]. In higher plants it is one of the proteins expressed in etiolated and illuminated leaves on the same level, which indicates that it function may be considered separately from the rest of the multiprotein complex. It was thus chosen as model small protein with dominant transmembrane helix for assessment of several methods for structure determination.
Experiments
Preparation of psbH
The PsbH protein of cyanobacterium Synechocystis
sp. PCC 6803 was expressed as a fusion protein with glutathione-S
transferase (GST) in E. coli [2]. We isolated the 15N labeled
PsbH protein in concentration of 1.1 mg/ml in presence of detergent octyl
glucoside (OG). We also isolated non-labeled protein for preliminary lipid
titration experiments measured by circual dichroism (CD) spectrometer.
Reconstitution and CD measurements
The liposomes were prepared by
reverse-phase evaporation technique from the thylakoid membrane lipids;
sulphoquinovosyl diaglyceride (SQDG), digalactosyl diglyceride (DGDG),
monogalactosyl diglyceride (MGDG) and phosphatidyl glycerol (PG). The most
favourable lipid, which induced complex protein folding, detected as formation
of the negative band approx. 222 nm in CD spectra, seemed to be PG. Very
similar changes were observed at higher concentration also in SQDG, however
folding of clearly different nature was achieved upon titration by DGDG. This
indicates that the protein folding may not be directly related to specific
binding of lipids, rather we observe two different types of folding in lipid
bilayers of two different properties.
NMR measurements
The CD measurements revealed folding of the PsbH
protein in detergent micelles after addition of sufficiant amount of lipid. We
added to each protein sample appropriate amount of the lipid to reach optimal
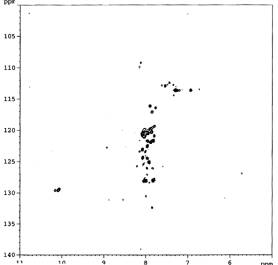
protein/ lipid ratio. Unfortunately NMR measurements showed a huge decrease of
signal and recording of the remaining 15N signals into the narrow area (figure
1). This would indicate very rigid lipid-protein micelles, which relax to fast
to be recorded.
Micelle destabilisation using sonication or
temperature increase led to only partial improvement, therefore we added into
the sample new detergents; CHAPS and digitonin. The simple addition of CHAPS or
digitonin did not destabilize micelles suficiently and we had to remove lipids
by dialysis. After dialysis signal recovered, moreover the new peaks indicated
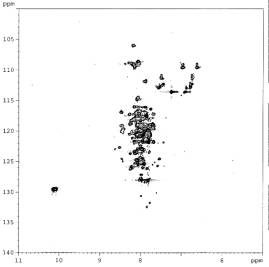
the further protein folding. The combination of digitonin and octyl glucoside
was determined as the most effective combination to induce apparent protein
folding (figure 2).
|
Figure 1 PsbH
protein after addition of PG |
Figure 2
PsbH OG with digitonin |
Crystallisation of fusion protein and X-ray diffraction
Fusion protein GST-psbH was
crystallized using standard procedures. Sufficiently
large crystals were obtained. The diffraction was, however, not measurable due
to problems of crystal cryoprotection. Experiments continue on improvement of
crystal quality of both the fusion protein and the protein itself.
[1] Komenda
J., Lupínková L. & Kopecký J., Eur. J. Biochem., 269 (2002) 610–619.
[2] Halbhuber Z., Petrmichlová Z., Alexciev
K., Thulin E. & Štys D., Protein Exp. Purif., 32-1 (2003) 18-27.