Phase analysis of soil sediments with regard to the presence of asbestos minerals
Zdeněk Jansa, Štěpánka Jansová, Ján Minár
New Technologies Research Centre, University of West Bohemia in Pilsen, Pilsen
The aim of this work is to summarize current knowledge on the extensive issue of asbestos occurrence in general and in the Pilsen Region of the Czech Republic, to establish a suitable methodology for detecting the presence of naturally occurring asbestos in soil deposits in a given location based on experimental analyses motivated by analyses in other countries, and to accurately identify individual types of asbestos from a series of samples. Twelve samples were evaluated as part of this work, and this paper presents a summary of them. [1,2]
The morphology and elemental composition of the studied samples were evaluated using scanning electron microscopy with an energy dispersive spectrum detector. Figure 1 shows examples of fibers resembling needles with very sharp ends, which morphologically corresponded to the amphibole group, and long, wavy fibers, which corresponded to the serpentine group.


Figure 1: Images of samples 3 and 5
The basic building block of the silicate structure of asbestos is the silicon-oxygen tetrahedron [SiO4]4-. Chrysotile, as a representative of the first group of asbestos - serpentines, is hydrated magnesium silicate and its stoichiometric chemical composition can be given as Mg3Si2O5(OH)4. However, it has been observed that the chemical composition of the fibrous phase is closely related to the composition of the surrounding rock matrix and can be highly variable, as can be seen in the overview of the summary formulas of asbestos compounds in Table 1. [3,4,5]
The chemical composition of minerals that make up the second group of asbestos—amphiboles—reflects the complexity of the environment in which they were formed and can vary considerably in terms of major and trace elements and other influences that contributed to their formation. Amphibole fibers can be considered a series of minerals in which one cation is gradually replaced by another. [6,7]
Table 1: Summarized formulas of asbestos compounds.
Chrysotile Mg3Si2O5(OH)4
Amosit e (Fe2, Mg)7Si8O22(OH)2
Crocidolite Na2(Fe, Mg)3Fe2Si8O22(OH)2
Antophylite (Mg, Fe2)7Si8O22(OH)2
Tremolite Ca2Mg5Si8O22(OH)2
Aktinolite Ca2(Mg, Fe2)5Si8O22(OH)2
The second method used was X-ray phase analysis. The samples were measured under identical conditions. The measurements were performed on a Panalytical X'Pert Pro powder diffractometer with a copper X-ray tube (Kα1 = 0.154 nm). An ultra-fast Pixcel semiconductor detector was used with evaluation in the High Score program. Standard symmetrical geometry with a Bragg-Brentano arrangement was used for the measurements.
The measurement range for all samples was set identically between 20 and 85° [2θ]. When evaluating the samples, it was found that the main diffraction lines are located within an angle of 40° [2θ], while other diffraction lines beyond this angle belong to the SiO2 phase. Therefore, a section ranging from 20° to a maximum of 40° [2θ] was selected on all diffractograms.
Figure 2 shows the diffraction patterns of samples 1 and 2, before and after annealing, with the identified phases marked in the range from 20 to a maximum of 40°[2θ]. The numerical designation of these phases corresponds to the numerical designation of the identified phases in Table 2.
Since the evaluation of the diffraction patterns of the unprocessed samples revealed a significant presence of organic components in the samples, all samples were annealed before further measurement. The samples were annealed at 530 °C for 4 hours and then cooled naturally. The temperature of 530 °C is below the thermal decomposition temperature of asbestiform minerals, so there was no loss of native information from the samples.
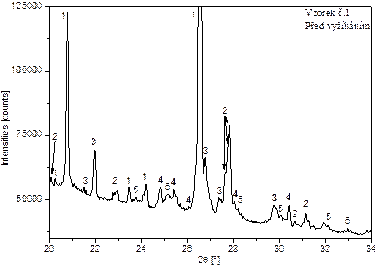
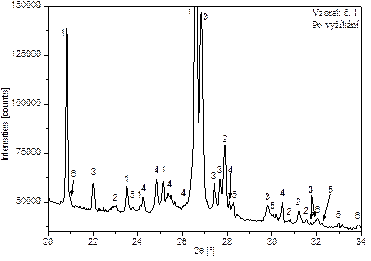
Figures 2: Evaluated diffractograms of sample 1 and 2 with identification of phases.
Table 2: Table of identified phases in soil sediment samples.
|
No |
Mineral |
Name of compound |
Reference code |
Chemical formula |
|
1 |
quartz |
silicon oxide |
01-089-8935 |
SiO2 |
|
2 |
--- |
magnesium silicate |
01-086-0433 |
Mg2(Si2O6) |
|
3 |
antofylit |
antofylite |
96-901-6382 |
Mg28Si32O96 |
|
4 |
chryzotil |
chryzotile |
96-101-0961 |
Si16Mg24O72 |
|
5 |
wollastonit |
calcium silicate |
01-072-2297 |
CaSiO3 |
|
6 |
--- |
hydrogen silicate |
00-031-0581 |
H2Si2O5 |
Scanning electron microscopy confirmed the presence of fibers that, from a morphological point of view, corresponded to both main groups of asbestos minerals, namely the serpentine and amphibole groups. X-ray diffraction identified individual phases in the samples and determined the exact type of asbestos minerals found.
After compiling the data from all analyses, we can say with certainty that chrysotile from the serpentine group is present in all evaluated samples. This is the least dangerous form of asbestos. Furthermore, we can say that the presence of anthophyllite from the amphibole group has been confirmed in all samples. Based on the morphology of the fibers, it is highly likely that crocidolite is also present in one sample, but this has not been confirmed by X-ray diffraction. The results of the asbestos found are shown in Table 3.
Tab 3: Summary table of identified asbestos types
|
Sample ID |
Chrysotile |
Anthophyllite |
Actinolite |
|
S1:sample 1-2 |
✓ |
✓ |
✗ |
|
S1:sample 3-4 |
✓ |
✓ |
✓ |
|
S2:sample 1′–8′ |
✓ |
✓(except 6′) |
✗ |
The combination of SEM analysis and X-ray diffraction provides a good set of tools for identifying asbestos. By gradually refining the measured diffraction pattern of the sample under investigation, it is possible to accurately determine the phases present, despite the complexity of the process.
1. F. Skácel, Z. Guschlová a V. Tekáč: Azbestová a minerální vlákna ve vnitřním ovzduší, Ústav plynárenství, koksochemie a ochrany ovzduší VŠCHT v Praze, Chemické listy 106, 961-970, 201
2. Lajčíková, M. Hornychová: Azbest v ovzduší a legislativní zajištění ochrany zdraví, Státní zdravotní ústav Praha, 55(3), 99-101, 2010
3. M. Novák: Mineralogický systém, prezentace [online – cit. 2021-12-06], Masarykova univerzita, available from: https://is.muni.cz/el/sci/podzim2011/G1061/Minera-I-system3a.pdf
4. C. E. Housecroft, A. G. Sharpe: Anorganická chemie, ISBN 978-80-7080-872-6
5. U.S. DEPARTMENT OF THE INTERIOR U.S. GEOLOGICAL SURVEY Asbestos: Geology, Mineralogy, Mining, and Uses by Robert L. Virta1, Open-File Report 02-149
6. O.C. Wells: Scanning Electron Microscopy, McGraw-Hill, New York (1974)
7. M.Kužvart, Z. Weiss, Jílové materiály, jejich nanostruktura a využití, Praha červen 2005, ISBN 80-246-0868-5