Properties of Schwertmannite: The Critical Role of Phase Purity
C. Pilloni1, V. Mameli2*, T. Kmječ3, V. Gajdošova4, C. Cannas2, D. Zákutná1*
1 Department of Inorganic Chemistry, Charles University, Hlavova 2030, Prague 2 128 40, Czech Republic
2Department of Chemical and Geological Sciences, University of Cagliari, Cittadella Universitaria S.P.
Monserrato Sestu Km 0.700, 09042 Monserrato, Italy
3Department of Low-Temperature Physics, Faculty of Mathematics and Physics, Charles University, V
Holešoviˇckách 2, 180 00 Prague, Czech Republic
4Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, 162 06 Prague 6, Czech Republic
*Correspondence: valentina.mameli@unica.it,
zakutnad@ill.fr
Schwertmannite, a poorly crystalline iron oxyhydroxysulphate, is an iron-bearing mineral that plays a pivotal role in various environmental processes, particularly in the treatment of acidic mine drainage [1]. Due to its ability to adsorb metal ions, anions, and its high surface area-to-volume ratio, schwertmannite has drawn significant attention as a potential medium for mitigating environmental contamination [2]. However, its poorly crystalline structure presents significant challenges in characterising its composition, making it difficult to detect and to quantify trace impurities. One such impurity is goethite, another iron mineral that can form under similar conditions due to higher thermodynamic stability [3]. Differentiating between schwertmannite and goethite in environmental or synthetic samples is crucial, as the presence of goethite affects the chemical reactivity and stability of schwertmannite, thereby altering its efficiency in ecological applications. The aim of this study is to characterise four synthetic samples of schwertmannite with different levels of goethite impurity. The presence was detected using a combination of Room-Temperature Powder X-ray Diffraction (RT-PXRD) and High-Temperature Powder X-ray Diffraction (HT-PXRD), Fourier Transform Infrared spectroscopy in Attenuated Total Reflectance mode (ATR-FTIR), and Thermogravimetric Analysis (TGA), with characteristic features in all the techniques. Notably, increasing precursor concentration led to decreased goethite content in the samples, as evidenced by the progressive disappearance of diffraction maxima observed from RT-PXRD (Fig. 1a). This is further confirmed by the presence of the hematite diffraction maxima after 400 °C in the samples richer in goethite (Fig. 1b, c). Interestingly, only magnetisation measurements provide information on the presence of goethite in the purest sample, demonstrating it as a powerful probe for this poorly crystalline system. These findings confirm that magnetic characterization based on Vibrating Sample Magnetometer (VSM) can serve as an effective tool for identifying goethite impurities in schwertmannite, thereby contributing to the knowledge of poorly crystalline iron materials, and highlighting the potential of magnetic techniques for enhancing our comprehension of these materials in both natural and engineered systems.
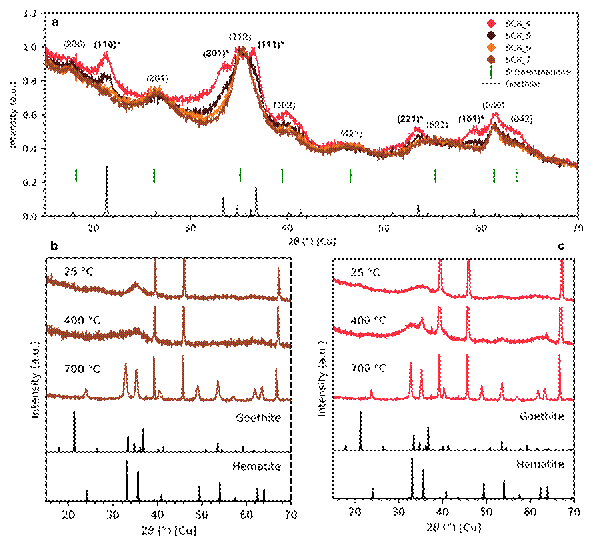
Figure 1 – a) room temperature powder x-ray diffraction pattern of all the samples, high-temperature powder x-ray diffraction pattern of: b) SCH4, sample richer in goethite, and c) SCH7, purest sample.
[1] J. M. Bigham, U. Schwertmann, S. J. Traina, R. L. Winland, and M. Wolf, ‘Schwertmannite and the chemical modeling of iron in acid sulfate waters’, Geochim. Cosmochim. Acta, vol. 60, no. 12, pp. 2111–2121, Jun. 1996, doi: 10.1016/0016-7037(96)00091-9.
[2] B. Marouane et al., ‘The potential of granulated schwertmannite adsorbents to remove oxyanions (SeO32−, SeO42−, MoO42−, PO43−, Sb(OH)6−) from contaminated water’, J. Geochem. Explor., vol. 223, p. 106708, Apr. 2021, doi: 10.1016/j.gexplo.2020.106708.
[3] P. Acero, C. Ayora, C. Torrentó, and J.-M. Nieto, ‘The behavior of trace elements during schwertmannite precipitation and subsequent transformation into goethite and jarosite’, Geochim. Cosmochim. Acta, vol. 70, no. 16, pp. 4130–4139, Aug. 2006, doi: 10.1016/j.gca.2006.06.1367.