Unraveling the B. pseudomallei Heptokinase WcbL:
From Structure to Drug Discovery.
M. Vivoli1, M.N. Isupov1, R. Nicholas1, A. Hill1, A.E. Scott2, P. Kosma3, J.L. Prior2, N.J. Harmer1
1Department of Biosciences, University of Exeter, Henry Wellcome Building, Stocker Road, Exeter EX4 4QD, UK.
2Defence Science and Technology Laboratory, Porton Down, Salisbury, Wiltshire SP4 0JQ, UK.
3University of Natural Resources and Life Sciences-Vienna, Muthgasse 18, 1190 Vienna, Austria.
n.j.harmer@exeter.ac.uk.
Bacterial cells display a variety of polysaccharides and glycoconjugates in their cell walls and external structures. Gram-negative bacteria utilize heptoses as part of their repertoire of extracellular polysaccharide virulence determinants. The polysaccharides of particular relevance for infection are the lipopolysaccharides (LPS) and capsular polysaccharides (CPS). The Gram-negative bacterium B. pseudomallei requires CPS for full virulence [1]. This pathogen is the causative agent of melioidosis, the most common cause of community-acquired septicemia in many regions of South-East Asia [2,3]. Mortality from septicemia ranges from 14% to 50%, depending on treatment, and up to 90% if untreated [4]. The organism is also considered to be a significant threat for bioterrorism [5] and is currently classed by the US Centers for Disease Control and Prevention as a tier-1 agent [6]. New treatments are being urgently sought by the USA and UK defense agencies. Disruption of heptose biosynthesis offers an attractive target for novel antimicrobials. A critical step in the synthesis of heptoses is their 1-O phosphorylation, mediated by kinases such as HldE or WcbL.

Figure 1. Schematic representation GDP-linked pathway for the capsular polysaccharide biosynthesis, highlighting the kinase activity of WcbL. S7P, sedoheptulose-7-phosphate, M7P, D-manno-α,β-D-heptose-7-phosphate; phosphorylation of D-α,β-D-heptose-7-phosphate to D-α-D-heptose-1,7PP (M7PP) using ATP.
Here, we present the structure of WcbL from Burkholderia pseudomallei. We report that WcbL operates through a sequential ordered Bi-Bi mechanism, loading the heptose first and then ATP. We show that dimeric WcbL binds ATP anti-cooperatively in the absence of heptose, and cooperatively in its presence. Modeling of WcbL suggests that heptose binding causes an elegant switch in the hydrogen-bonding network, facilitating the binding of a second ATP molecule.
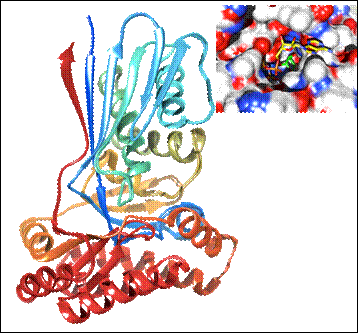
Figure 2. Monomeric crystal structure of WcbL at 1.76 Å. The backbone is shown in cartoon representation, rainbow colored from red (N terminus) to blue (C terminus). Molecular surface of WcbL coloured by charge density (red: negative; blue: positive) with bound AMPPNP and D-mannose.
Finally, we screened a library of drug-like fragments, identifying hits that potently inhibit WcbL. Based on the structural and kinetic data, we have proposed a mechanism for the action of WcbL, and show that small drug-like fragments are competent in binding to the active site and inhibiting WcbL in competition with ATP. Given that WcbL knockouts present a very strong phenotype, these data strongly suggest that WcbL would be an excellent target for adjunct therapies to prevent the formation of protective surface polysaccharides in Gram-negative bacteria.