Crystal structure analyses of dipeptidyl peptidase 11 from Porphyromonas gingivalis
Y. Sakamoto1, Y. Suzuki2, I. Iizuka1, C. Tateoka1, S. Roppongi1, M. Fujimoto1, K. Inaka3, H. Tanaka4, M. Yamada5, K. Ohta5, H. Gouda6, T. Nonaka1, W. Ogasawara2, N. Tanaka6,7
1School of Pharmacy, Iwate Medical University, Iwate 028-3694, Japan
2Department of Bioengineering, Nagaoka University of Technology, Niigata 940-2188, Japan
3Maruwa Foods and Biosciences Inc., Nara 639-1123, Japan
4Confocal Science Inc., Tokyo 101-0032, Japan
5Japan Aerospace Exploration Agency, Ibaraki 305-8505, Japan
6School of Pharmacy, Showa University, Tokyo 142-8555, Japan
7Center for Molecular Analysis, Showa University, Tokyo 142-8555, Japan
ntanaka@pharm.showa-u.ac.jp
Periodontitis is a bacterially induced inflammatory disease that destroys the periodontal tissues, eventually leading to tooth loss. Porphyromonas gingivalis, a Gram-negative, anaerobic bacterium, is a major pathogen associated with the chronic form of periodontitis. Because P. gingivalis is an asaccharolytic bacterium that gains its metabolic energy by fermenting amino acids, P. gingivalis secretes various proteases/peptidases that are capable of digesting external proteins into peptides. P. gingivalis utilises di- and tripeptides, instead of single amino acids, as sources of carbon and energy. Therefore, peptidases of P. gingivalis that provide di- and tripeptides are essential for the metabolism of the bacterium, and much attention has been paid to dipeptidyl peptidases (DPPs) from P. gingivalis. Recently, novel DPPs, DPP5 (PgDPP5), DPP7 (PgDPP7) and DPP11 (PgDPP11), have been identified from P. gingivalis [1-3]. The P. gingivalis DPPs, PgDPP5 has been classified as clan SC, family S9 in the MEROPS database, while PgDPP7 and PgDPP11 have been assigned to another type of serine peptidase family, S46 in clan PA. Whereas PgDPP7 exhibits a broad substrate specificity for both aliphatic and aromatic residues at the P1 position (NH2-P2-P1-P1’-P2’-…, where the P1-P1’ bond is the scissile bond), PgDPP11 exhibits a strict substrate specificity for acidic residues (Asp/Glu) at the P1 position.
The S46 peptidases are widely distributed in anaerobic Gram-negative species, but they are not found in mammals. Therefore, the family S46 peptidases may represent ideal targets for novel antibiotics. Recently, the first three-dimensional structure of a S46 peptidase was determined for dipeptidyl peptidase BII (DAP BII) from Pseudoxanthomonas mexicana WO24 [4]. The study revealed that DAP BII is a homodimer and each subunit contains a peptidase domain including a double β-barrel fold that is characteristic of the chymotrypsin superfamily, as well as an unusual α-helical domain that regulates the exopeptidase activity of DAP BII. Although the overall structure, the molecular basis of the exopeptidase activity, and the catalytic mechanism of the S46 peptidase have been revealed by the crystal structure analyses of DAP BII [4], determinants for the substrate specificity of S46 peptidases at the atomic level remain to be fully elucidated.
In this study, we present the crystal structure of PgDPP11 (Fig. 1), a member of the S46 peptidase family. The crystal structure analyses, in silico docking studies, and site-directed mutagenesis studies clearly explain the molecular basis of the Asp/Glu specificity of PgDPP11 [5], which is determined by the conserved Arg residue in the S1 subsite (Fig. 2).
High-resolution diffraction data obtained from a space-grown crystal enabled us to identify two potassium ion-binding sites in the catalytic domain of PgDPP11 because the present crystallisation conditions contained 0.16 M tri-potassium citrate in the reservoir solution. One was found at the N-terminal amino group binding site, and the other was found at the bottom of the S1 subsite. The former potassium ion was coordinated by the side chains of Asn218 and Asp672 and a water molecule and was also stabilised by a cation-pi interaction with the indole ring of Trp219. Similar cation-pi interaction was observed between the indole ring of Trp216 in DAP BII and the N-terminal amino group of bound peptide [4]. The space grown crystal was obtained using a counter-diffusion crystallisation method under a microgravity environment in the Japanese Experimental Module “Kibo” at the International Space Station (ISS) [6].
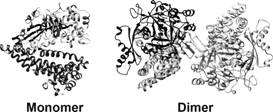
Figure 1. Overall structure of PgDPP11.
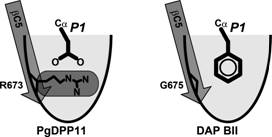
Figure 2. Schematic diagrams of the S1 subsites of S46 peptidases.
1. A. Banbula, J. Yen, A. Oleksy, P. Mak, M. Bugno, J. Travis, J. Pogtempa, J. Biol. Chem., 276, (2001), 6299.
2. Y. Ohara-Nemoto, Y. Shimoyama, S. Kimura, A. Kon, H. Haraga, T. Ono, T. K. Nemoto, J. Biol. Chem., 286, (2011), 38115.
3. Y. Ohara-Nemoto, S. M. Rouf, M. Naito, A. Yanase, F. Tetsuo, T. Ono, T. Kobayakawa, Y. Shimoyama, S. Kimura, K. Nakayama, K. Saiki, K. Konishi, T. K. Nemoto, J. Biol. Chem., 289, (2014), 5436.
4. Y. Sakamoto, Y. Suzuki, I. Iizuka, C. Tateoka, S. Roppongi, M. Fujimoto. K. Inaka, H. Tanaka, M. Masaki, K. Ohta, H. Okada, T. Nonaka, Y. Morikawa, K. T. Nakamura, W. Ogasawara, N. Tanaka, Sci. Rep., 4, (2014), 4977.
5, Y. Sakamoto, Y. Suzuki, I. Iizuka, C. Tateoka, S. Roppongi, M. Fujimoto, K. Inaka, H. Tanaka, M. Yamada, K. Ohta, H. Gouda, T. Nonaka, W. Ogasawara, N. Tanaka, Sci. Rep., 5, (2015), 11151.
6. S. Takahashi, K. Ohta, N. Furubayashi, B. Yan, M. Koga, Y. Wada, M. Yamada, K. Inaka, H. Tanaka, H. Miyoshi, T. Kobayashi, S. Kamigaichi, J. Synchrotron Radiat., 20, (2013), 968.
We thank Drs. Y. Yamada and N. Matsugaki of the Photon Factory and Dr. A. Higashiura, K. Hasegawa and E. Yamashita of SPring-8 for their help with data collection at the synchrotron facilities. This study was supported in part by the Platform for Drug Discovery, Informatics, and Structural Life Science (to N.T.), the Program for the Strategic Research Foundation at Private Universities from the MEXT of Japan (to N.T. and Y.Sa), and a Grant-in-Aid for Scientific Research(C) (to Y.Sa.). This study was also supported in part by “High-Quality Protein Crystal Growth Experiment on JEM” promoted by JAXA (Japan Aerospace Exploration Agency) (to Y.Sa.). The Russian Spacecraft “Progress” and “Soyuz” provided by the Russian Federal Space Agency were used for space transportation.