Structural investigations of the Interleukin-5 receptor ectodomain
J-P. Scheide, T.D. Mueller
Department of Molecular Plant Physiology and Biophysics,
Julius-von-Sachs Institute,
University of Wuerzburg, Wuerzburg, Germany
jan-philipp.scheide@uni-wuerzburg.de
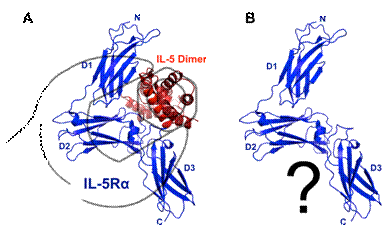
Interleukin-5 (IL-5) is a hematopoietic cytokine produced mainly by T-lymphocytes controlling a multitude of functions in the immune system. It is mainly known as a key regulator of eosinophilic granulocytes (eosinophils). Here IL-5 controls almost any aspect of the eosinophil’s life, from differentiation and maturation of eosinophil progenitor cells, migration into target tissues, to their proliferation and activation. Eosinophils are a part of the immune response for the defence of antigens and are usually activated during helminth infection, allergic diseases and asthma. Upon their activation pro-inflammatory mediators such as leukotriene, histamine and reactive oxygen species are released in a process called degranulation thereby leading to the known allergy symptoms. Signalling of IL-5 occurs in a sequential receptor binding mechanism. IL-5 binds first to its specific a chain the IL-5 receptor (IL-5Ra) and then recruits the common beta chain (bc, CD131) to form a ternary complex mainly activating the JAK/STAT signalling cascade. As IL-5 is the key regulator of eosinophils the cytokine has become a highly interesting target for pharmaceutical intervention. The molecular mechanism of IL-5 receptor activation is not fully known yet. We have previously determined the structure of IL-5 bound to the ectodomain of the IL-5 receptor IL-5Rα (binary complex) by crystallography [1], which provides insights into the first step of receptor activation. From a structure-function analysis we found hints that the novel wrench-like architecture of the IL-5 receptor is possibly preformed (Fig. 1). This notion has great impact for the design of small molecule-based IL-5 inhibitors as such molecules will target the structure of the unbound IL-5Rα, which must be known to successfully address the receptor. Our goal is therefore to determine the structure of the IL-5Ra ectodomain in its unbound conformation. We were able to optimize the expression/purification for the ectodomain protein to yield highly pure protein suitable for structural characterization. We have started screening 1000+ crystallization conditions to obtain crystals of IL-5Ra for structure determination by X-ray diffraction. First crystals have been obtained and optimization is in progress (Fig.2).