Crystallization and Structure Determination of human Aldehyde Oxidase and Complexes
C. Coelho1, T. Santos-Silva1, A. Foti2, S. Leimkühler2 and M.J. Romão1
1UCIBIO@REQUIMTE – Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829-516 Caparica, Portugal
2Institute of Biochemistry and Biology, Department of Molecular Enzymology, University of Potsdam, D-14476 Potsdam, Germany
c.coelho@fct.unl.pt
Aldehyde oxidase (AOX) is a xanthine oxidase (XO) related enzyme with emerging importance in the metabolism of drugs and xenobiotics [1]. The hAOX holoenzyme is a homodimer (2x150 kDa) and each monomer is constituted by three domains: the N-terminal domain with two distinct [2Fe-2S] clusters (FeSI and FeSII), the central FAD binding domain and the C-terminal catalytic Moco domain. We report the first crystal structure of human AOX in the substrate-free form (2.6Å resolution) and in complex with the substrate phthalazine and the inhibitor thioridazine (2.7Å resolution) (Fig 1-A) [2].
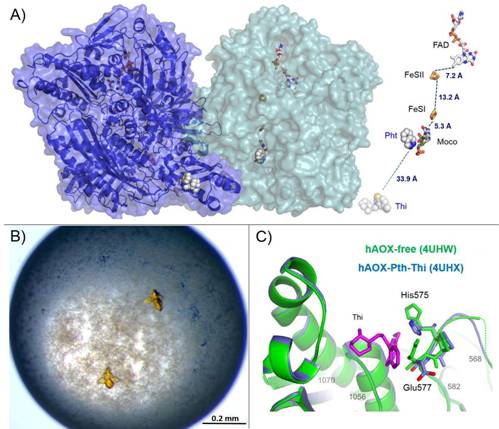
Figure 1. (A) Surface representation of the hAOX crystal structure and different protein cofactors and distances (FAD, FeSII, FeSI and Moco). The phthalazine (Pht) and thioridazine (Thi) molecules from different monomers (light green and blue) are represented in space-filling mode; (B) Crystals of hAOX with approximate dimensions of 0.1x0.15x0.15 obtained using polyethylene glycol 4000; (C) Close-up of the thioridazine molecule (pink) binding site pocket with the structures of hAOX-free (green) and hAOX-Pht-Thi (blue) superimposed.
Crystals of hAOX were obtained through the vapour-diffusion technique using polyethylene glycol 4000 as precipitant. The crystals are very sensitive to manipulation and grew to their maximum size within 24 h (Fig 1-B). Extensive trials and modified protocols were performed in order to promote better crystallization conditions, improve crystal quality and allow co-crystallization and soaking experiments with bound substrate and/or inhibitors.
Crystals with bound molecules were only obtained when using large amount of the substrate phthalazine as co-crystallization agent, followed by soaking of the obtained crystals with a thioridazine hydrochloride harvesting solution. Analysis of the protein active site combined with steady-state kinetics highlight the unique features that characterize hAOX as an important drug-metabolizing enzyme, which include binding and substrate orientation at the active site [2].
The structure of the complex with the non-competitive inhibitor thioridazine revealed a new, unexpected and fully occupied inhibitor-binding site, structurally conserved among mammalian AOXs and XO (Fig 1-C). The new structural insights into the catalytic and inhibition mechanisms of human AOX now reported will be of great value for the rational analysis of clinical drug interactions involving inhibition of AOX1 as well as for predicting and designing AOX-stable putative drugs [2].
1. M.Terao, M.J.Romão, S.Leimkühler, M.Bolis, M.Fratelli, C.Coelho, T.Santos-Silva and E.Garattini “Structure and function of mammalian aldehyde oxidases”, ARCH. TOXICOL, 90 (2016): 753-780. doi: 10.1007/s00204-016-1683-1
2. C. Coelho, A .Foti, T. Hartmann, T. Santos-Silva, S. Leimkühler and M.J. Romão “Structural insights into xenobiotic and inhibitor binding to human aldehyde oxidase” NATURE CHEM. BIOL., 11(10) (2015): 779-83. doi: 10.1038/nchembio.1895