In-situ observation of protein crystal growth under magnetic quasi-microgravity conditions on earth
M. Tanokura1, A. Nakamura1, N. Hirota2, H. Wada1,2
1Department of Applied Biological Chemistry, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-8657, Japan
2National Institute for Materials Science, 3-13 Sakura, Tsukuba, Ibaraki 305-0003, Japan
amtanok@mail.ecc.u-tokyo.ac.jp
Protein crystallization in space is assumed to proceed in purely diffusional manner without gravity-induced convection [1]. As a result, protein crystals are expected to be formed free of defects and/or impurities. Proteins as well as protein solutions are usually diamagnetic, so that a similar effect on convection should be possible by magnetic force. Magnetic force (Fm) is proportional to the product of magnetic field (B) and magnetic field gradient (dB/dz), as given in the following equation (1):
|
|
(1) |
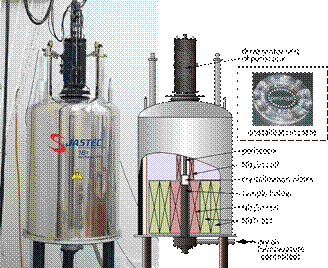
where m0 is the vacuum permeability, m is the mass, cm is the mass susceptibility, Bz and dBz/dz is the magnetic flux density and gradient of magnetic field, respectively, in vertical direction. A diamagnetic substance is levitated or its motion is impeded when Fm is equal or close to gravity. Therefore, a magnetic field and a magnetic field gradient have been utilized to obtain high quality protein crystals, in which magnetic orientation of crystals and reduction in natural convection contribute mainly to the crystal quality improvement [2]. Using this technology we have developed a high-throughput and high-quality protein crystal growth system with strong magnetic force that enables to cancel out the gravity of a water droplet. This system consists of a superconducting magnet, protein crystallization plates, a temperature controller, and equipment for in-situ observation (Fig. 1) [3].
The system developed contains two groups of superconducting coils; the top coil of Nb3Sn generates magnetic fields downward, while the bottom coils of NbTi and Nb3Sn generate magnetic fields upward; these coils are operated in persistent mode. The maximum magnetic field generated reaches 16 T. Fm and dBz/dz become largest between the two groups of coils, creating a reduced-gravity zone where the crystallization plates are placed (Fig. 1).
Since the crystallization plates are placed in the magnet bore, we cannot know if crystallization has occurred until we examine the cell outside of the bore. Then, we not only interrupt the crystallization process, but also change the whole gravity conditions. In terms of efficiency, it is highly desirable not to interrupt the crystallization process by taking out the crystallization plates from the bore. It is direct observation that eliminates these problems.
We have made up an optical observation probe which can look into the crystallization plates in the magnet bore, enabling in-situ observation of the crystallization process. The optical probe has a 3-D adjustable focusing mechanism with enough high resolution of a few microns (Fig. 1).
Protein crystallization has been carried out through the sitting drop vapour diffusion method. In the system, we can stack 10 crystallization plates, each having 12 solution reservoirs and 24 crystallization drop wells, enabling us to examine 240 conditions in one experiment. The temperature around the crystallization plates is controlled by the dry air between 4 and 20°C within ± 0.1°C.
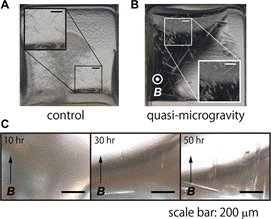
In this study, we have conducted crystallization experiments using two protein samples, a GFP (green fluorescent protein)-like protein and a zinc protease. In the case of the GFP-like protein, triangular pyramid-shaped crystals appeared within 12–24 hours after the initiation of vapour diffusion, exhibiting magnetic orientation. X-ray diffraction experiments indicated that the crystallographic c-axis was parallel with the direction of the magnetic field. Needle-shaped thin crystals of the zinc protease also showed magnetic orientation (Fig. 2). The long side of crystals was aligned along the c-axis which is parallel with the direction of the magnetic field. The crystals were grown 1–2 days. X-ray diffraction measurements showed that these protein crystals obtained in the magnetic quasi-microgravity had better crystallographic quality and less variation in quality than crystals grown without the magnetic field.
|
|
|
Figure 1. Developed system for protein crystallization under magnetic quasi-microgravity conditions. |
Figure 2. Crystals of a zinc protease in the control experiment (A) and grown under the magnetic quasi-microgravity conditions (B). (C) Crystal growth in the quasi-microgravity. |
1. B. Lorber, Biochim. Biophys. Acta, 1599, (2002), 1–8.
2. A. Nakamura*, J. Ohtsuka*, K. Miyazono*, A. Yamamura, K. Kubota, R. Hirose, N. Hirota, M. Ataka, Y. Sawano, M. Tanokura, Cryst. Growth Des., 12, (2012), 1141–1150. (* equal contribution)
3. A. Nakamura, J. Ohtsuka, T. Kashiwagi, N. Numoto, N. Hirota, T. Ode, H. Okada, K. Nagata, M. Kiyohara, E. Suzuki, A. Kita, H. Wada, M. Tanokura, Sci. Rep., 6, (2016), 22127.
This work was supported by “Development of Systems and Technology for Advanced Measurement and Analysis (Program-S)” Fund of Japan Science and Technology Agency.