A comparative study on the diffraction quality of protein crystals obtained using the cross-diffusion microbatch and sitting-drop vapor diffusion methods
H. Hou, B. Wang, S. Y. Hu, Y. Liu, D. C. Yin
School of Life Sciences, Northwestern Polytechnical University, Xi’an 710072, Shaanxi, P. R. China
houhai@nwpu.edu.cn
Improving the crystal quality of a protein is always an important target for structure determination using X-ray diffraction. In this project, we present a systematic quality comparison of protein crystals grown using the cross-diffusion microbatch (CDM) method and the standard sitting-drop vapor-diffusion method. Crystallization conditions for eleven different proteins were screened using these two methods, and the crystals of all conditions were checked in terms of the resolution limit and mosaicity. It was found that crystals grown in the plate using the CDM method exhibit better morphology and higher crystal quality than crystals obtained using the standard sitting-drop vapor-diffusion method. X-ray diffraction tests show that the CDM method is indeed a practical and useful method for obtaining high-quality protein crystals to reduce the workload associated with both protein crystallization screening and optimization.

Figure 1. Comparison of images of the crystallization plate using the CDM method and the SDVD method.
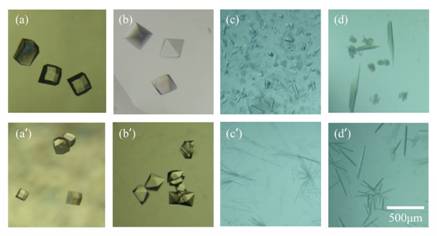
Figure 2. Typical morphology of crystals grown in the CDM and the SDVD crystallization plate.
A crystallization plate using the CDM method is shown in Fig. 1 [1]. (a) Image of crystallization plate using the traditional sitting drop vapor-diffusion (SDVD) method. (b) Image of crystallization plate using the cross-diffusion microbatch (CDM) method. The size of this plate is made to be compatible with the SBS standard plate. This crystallization plate is similar to the traditional SDVD plate, except for the material and the reservoir, which was replaced by more pits to increase the number of protein concentrations or pH gradients.
Fig. 2 shows some typical images [(a)-(d), crystals grown in a CDM crystallization plate; (a')-(d'), crystals grown in a SDVD crystallization plate]. The crystals grown in the CDM crystallization plate looked better than the crystals grown in the SDVD crystallization plates [2]. Some defective crystals were obtained from the SDVD crystallization plate. The crystal morphological comparison showed that the crystals grown in CDM crystallization plates displayed relatively better morphology than crystals grown in SDVD crystallization plates. (a), (aʹ) Lysozyme; (b), (bʹ) Proteinase K; (c), (cʹ) α-Chymotrypsinogen A II; (d), (dʹ) Catalase.
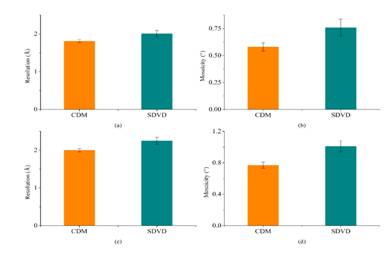
Figure 3. A comparison of the resolution limits of all the "Diffraction data" quality crystals obtained from the CDM crystallization plate and the SDVD crystallization plate
Fig. 3 exhibits a comparison of the resolution limits of all the "Diffraction data" quality the crystals obtained from the CDM and the SDVD crystallization plate [2]. (a) In terms of the resolution limit of the proteinase K crystals, the results demonstrated a significant difference between the two groups (P <0.05). (b) A comparison of the mosaicity of proteinase K. The results demonstrated significant difference between the two groups (P <0.05). (c) A comparison of the resolution lysozyme crystals obtained in the CDM and the SDVD crystallization plate. The difference between two groups was significant (P <0.05). (d) A comparison of the mosaicity of lysozyme crystals. The results showed an extremely significant difference between the two groups (P <0.01). Therefore, the CDM crystallization plate clearly demonstrated an improvement in both resolution and mosaicity compared with the SDVD method.
In conclusion, it was found that the CDM method can improve the quality of protein crystals compared with the conventional SDVD method. This crystallization screening method could be suitable for routine protein crystallization.
1. R. Q. Chen, D. C. Yin, Y. M. Liu, Q. Q. Lu, J. He, Y. Liu, Acta Cryst., D70, (2014), 647.
2. H. Hou, B. Wang, S. Y. Hu, J. Z. Wang, P. F. Zhu, Y. Liu, M. Y. Wang, D. C. Yin, CrystEngComm., 17, (2015), 5365.
This work was supported by National Natural Science Foundation of China (Grant No. 31200551).