The fine-tuned machinery of O2-tolerant [NiFe] hydrogenase
Andrea Schmidt, Jacqueline Kalms and Patrick Scheerer*
Institut für Medizinische Physik und Biophysik (CC2), Group Protein X-ray Crystallography & Signal Transduction, Charité - Universitätsmedizin Berlin, Charitéplatz 1, D-10117 Berlin, Germany
*patrick.scheerer@charite.de
Hydrogenases are metalloenzymes catalyzing the heterolytic splitting of hydrogen into protons and electrons. In all three domains of life hydrogenases are domiciled, but only a small subgroup of [NiFe] hydrogenases evolved the feature of hydrogen conversion under aerobic conditions. For enabling the aerobic hydrogen oxidation in [NiFe] hydrogenases, multiple adaptable pathways have been evolved. Structural investigations on this biological machine might lead to new developments in the field of renewable energy technologies [1].
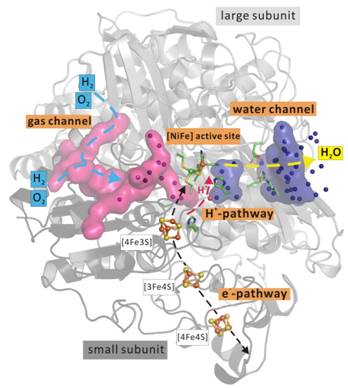
The membrane-bound [NiFe] hydrogenase (MBH) of Ralstonia eutropha (R.e.) is one of the best investigated typical O2-tolerant hydrogenases. Several crystal structures of the MBH R.e. as wildtype or with multiple substitutions in different redox states reveal a highly fine-tuned interplay between pathways and channels that lead to a perfect transport of reagents and products to and from the active site [2, 3]. For hydrogen splitting the [NiFe] active site of MBH R.e. requires the delivery of hydrogen via a hydrophobic gas channel. Hydrogen oxidation liberates electrons which are guided via an electron pathway to an electron acceptor. Subsequently, the electrons enter the quinone pool of the respiratory chain as reduction power for the cell [2]. Under aerobic conditions additionally the [NiFe] active site has to reduce the attacking oxygen to water with 4 e- and 4 H+. On that account the electron pathway, consisting of three [FeS] clusters, has to operate bidirectional. A unique [4Fe3S] cluster proximal to the active site is mainly involved in this switch. This [4Fe-3S] cluster undergoes redox-dependent reversible transformations, namely iron-swapping between a sulfide and a peptide amide N. For proton delivery several pathways close to the active site have been investigated and introduce new questions that might be answered by investigative methods e.g. neutron diffraction. The gas channel that is supplying also the inhibitory oxygen has been adapted especially in quantity and size to remain the hydrogenase activity for the system [4]. Water molecules produced under oxygen reduction are released through a new water channel. This complex system is still not completely understood and moreover sensitive to X-rays. Consequently a near radiation-damage free technique, the free-electron laser (e.g. LCLS, Stanford, USA), has been used to gain further insights into the functionality of this enzyme.
1. Fritsch, J., Lenz, O., Friedrich, B. (2013). Structure, function and biosynthesis of O2-tolerant hydrogenases. NatRevMicrobiol. 2, 106-114.
2. Fritsch, J., Scheerer, P., Frielingsdorf, S., Kroschinsky, S., Friedrich, B., Lenz, O., Spahn, CM. (2011). The crystal structure of an oxygen-tolerant hydrogenase uncovers a novel iron-sulfur centre. Nature 479 (7372), 249-52.
3 Frielingsdorf S, Fritsch J, Schmidt A, Hammer M, Löwenstein J, Siebert E, Pelmenschikov V, Jaenicke T, Kalms J, Rippers Y, Lendzian F, Zebger I, Teutloff C, Kaupp M, Bittl R, Hildebrandt P, Friedrich B, Lenz O§, and Scheerer P§. (2014). Reversible [4Fe-3S] cluster morphing in an O(2)-tolerant [NiFe] hydrogenase. Nat Chem Biol. 10, 378-385.
4. Kalms, J., Schmidt, A., Frielingsdorf, S., van der Linden, P., von Stetten, D., Lenz, O., Carpentier, P., Scheerer, P. Gas transport through a hydrophobic tunnel in O2-tolerant membrane-bound [NiFe] hydrogenase from Ralstonia eutropha. Angew Chem Int Ed Engl. 2016, doi: 10.1002/anie.201508976.