Thermodynamic stabilisation, entropy, and crystallisation of proteins
P. Stavros1, G. Nounesis1, E. Saridakis2
1Biomolecular Physics Laboratory, INRASTES,, National Centre for Scientific Research “DEMOKRITOS”, Athens 15310, GREECE
2Structural and Supramolecular Chemistry Laboratory, Insitute of Nanoscience and Nanotechnology, National Centre for Scientific Research “DEMOKRITOS”, Athens 15310, GREECE
e.saridakis@inn.demokritos.gr
When molecules previously in solution form a crystalline phase, their entropy is reduced. The crystallization driving force must then come either from the enthalpy of the intermolecular interactions in the crystal, or from a gain of entropy of the solvent. There is indeed often an entropy gain upon crystallisation, since a large proportion of the water molecules previously forming ordered hydration shells around the macromolecules are excluded from the crystal. The importance of both entropic and enthalpic effects for crystallisation suggests that changes in these parameters in the pre-crystallisation solution, brought about by the precipitant and buffer, will be crucial to the outcome.
High-accuracy Differential Scanning Microcalorimetry was used to measure the thermodynamic parameters of temperature-driven unfolding of two globular proteins, lysozyme and ribonuclease A, in various salt solutions. The salts were categorised into those that were conducive to crystallisation of the protein and those that were not. Both the Free Energy (ΔG) of unfolding at different temperatures and salt concentrations and its breakdown into entropic and enthalpic contributions were obtained.
It was found that even fairly low -by crystallisation standards- salt concentrations had very large effects on thermodynamic parameters. High concentrations of salts conducive to crystallisation stabilised the native folded forms of proteins, whereas high concentrations of salts that did not crystallise them tended to destabilise them. Considering the ΔH and TΔS contributions to the ΔG of unfolding separately, high concentrations of crystallising salts were found to enthalpically stabilise and entropically destabilise the protein, and vice-versa for the non-crystallising salts (Fig. 1).
These observations suggest an explanation, in terms of protein stability and entropy of hydration, of why some salts are good crystallisation agents for a given protein and others are not. This in turn provides theoretical insight into the process of protein crystallisation, possibly suggesting ways of predicting and controlling it.
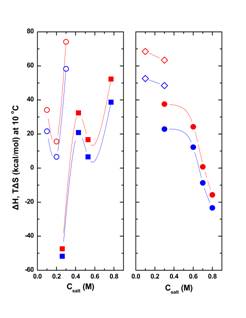
Figure 1. Enthalpic (ΔH, red) and entropic (TΔS, blue) contributions to the thermodynamic stability of lysozyme in 50 mM sodium acetate buffer (pH 4.5) at 10°C. Left panel: crystallising salts NaCl (full squares) and Na2SO4 (empty circles). Right panel: non-crystallising salts (NH4)2HPO4 (full circles) and Li2SO4 (empty diamonds).