Optimization of Crystallization using Dialysis Combined with Temperature Control
M. Budayova-Spano1,2,3*, N. Junius3,1,2, E. Oksanen4, J-L. Ferrer3,1,2
1Univ. Grenoble Alpes, 2CNRS,
3CEA, IBS, F-38044 Grenoble, France
4ESS, Lund, Sweden
*monika.spano@ibs.fr
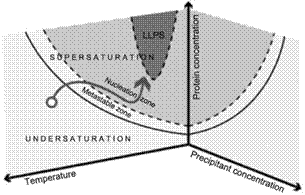
The crystallization process includes both thermodynamic and kinetic features in multidimensional phase spaces, and our understanding is based on a crystallization phase diagram, represented in a simplified form in Figure 1. Thermodynamic data are the solubility curves, the presence of metastable phases, polymorphs, liquid-liquid separation… They depend on multiple parameters such as temperature, pH, solvent, impurities, etc. In addition, kinetic trajectories in the phase diagram are relevant to control most of the final properties of the synthesized crystals. The path followed in the diagram controls the nucleation and growth of the crystals, and thus their number, size, and morphology.
Two new and emerging uses result in specific challenges for crystallization of proteins. In both, precise control of crystal size is essential. New approaches to serial (time-resolved) crystallography, where crystals in the 1-20 μm size are used to solve structures including those/structures of short-lived intermediates with reactions initiated by light or rapid mixing. Serial crystallographic methods are being increasingly used at synchrotron sources (serial synchrotron crystallography) due to advances in micro- and nano-focus beamlines, as well as at rapidly developing ultra-bright free-electron laser sources (serial femtosecond crystallography), enabling structure determination of previously intractable proteins. At the other extreme are the requirements of the next-generation flagship neutron sources, such as the ESS (European Spallation Source, Lund). Because neutrons interact very weakly with matter, much larger, and ideally cubic crystals are needed with volumes of > 0.01 mm3 (i.e. 200 μm on a side) for neutron crystallography in the future.
We have developed an apparatus and a method for the optimization of crystal growth using precise temperature control and dialysis combined with real-time visualization. Several neutron crystallography targets are being investigated on the developed system and the positive results already obtained indicating that the control of crystal growth does not compromise the diffraction quality, and rather improves it. The goal of this lecture is to provide the audience with related protocols using a thorough knowledge of the phase diagram and demonstrate how to select the starting position and kinetic pathway in optimizing the crystallization experiments.