Characterization of immune modulatory protein complex CD160-HVEM participating in bidirectional signalling pathways
I. Nemčovičová1,3, M. Nemčovič2, M. Kúdelová1, D. Zajonc3
1 Biomedical Research Center and 2 Institute of Chemistry, Slovak Academy of Sciences, Bratislava
2 Division of Cell Biology and Immune Regulation, La Jolla Institute, California, USA
viruivka@savba.sk
The majority of biological events involve the action of one macromolecule on another, thus triggering a series of recognition, signalling and modification events. The details of such macromolecular interactions are critical to our understanding of biological function and bestow greater knowledge than the three-dimensional structures of single macromolecules. Although substantial progress has been made in macromolecular docking, it still remains difficult to predict the mode of interaction between macromolecules even when the structures of the interacting partners are known. Natural killer (NK) cells express multiple activating receptors that, upon engagement, result in the rapid release of cytolytic and antiviral effectors required for host defence, notably against herpes viruses (β-herpesvirus cytomegalovirus, CMV). The host is protected from the powerful inflammatory mediators produced by NK cells through the action of inhibitory receptors. It was shown that the UL144 mimic of herpesvirus entry mediator (HVEM) from the CMV binds exclusively to B and T lymphocyte attenuator (BTLA) but not to CD160, resulting in inhibition of NK cells. HVEM and the two Ig-superfamily member receptors that bind HVEM, CD160 and BTLA, are all expressed on NK cells. Here, we show the molecular characterization and preliminary crystallographic analysis of CD160 and HVEM and therefore CD160-HVEM complex formation. CD160 is a 27 kDa glycoprotein which was initially identified with the monoclonal antibody BY55. Its expression is tightly associated with peripheral blood NK cells. The cDNA sequence of CD160 predicts a cysteine-rich, glycosylphosphatidylinositol-(GPI)-anchored protein of 181 amino acids with a single Ig-like domain weakly homologous to KIR2DL4 molecule. It was found that HVEM preferentially engages CD160 trimer to costimulate activation, while a viral ortholog of HVEM specifically binds to BTLA to suppress this signalling. CD160 antigen is a protein that in humans is encoded by the CD160 gene. We have found that CD160 is expressed at the cell surface as a tightly disulphide-linked multimer. The homology model of atomic structure of CD160 antigen domain [Fig.1] shows cysteine-rich region that was found to be responsible for CD160 tight-timer formation even under reduced conditions. The crystallization of multimeric CD160-HVEM complex was accessed by advanced macromolecular crystallization methods while non-reducing conditions. CD160 trimer forms stable complex with HVEM, while monomeric form refused to binds its cognate ligand. Taken together, regulation of CD160 bidirectional binding may represent a common mechanism of immune regulation targeted by multiple pathogens, which by extension is a potential target for therapeutic manipulation.
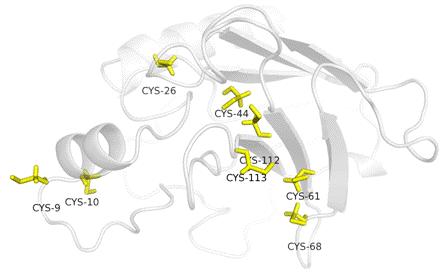
Figure 1. Homology model of CD160 structure with cysteine-rich intermolecular network.
IN is Marie Curie Fellow financed by Programme SASPRO, co-funded by European Union and the Slovak Academy of Sciences. The authors gratefully acknowledge the contribution of the Slovak Research and Development Agency under the project APVV-14-0839 and the contribution of the Scientific Grant Agency of the Slovak Republic under the grant 2/0103/15.