Purification and Crystallization of an Antigenic Outer-Membrane Protein from Salmonella Typhi
H.-H. Guan1, M. Yoshimura1, P. Chuankhayan1, C.-C. Lin1, N.-C. Chen1,2, C.-J. Chen1,2,3
1Life Science Group, Scientific Research Division, National Synchrotron Radiation Research Center, 101 Hsin-Ann Road, Hsinchu 30076, Taiwan
2Institute of Biotechnology, National Cheng Kung University, Tainan City 701, Taiwan
3Department of Physics, National Tsing Hua University, Hsinchu 30034, Taiwan
cjchen@nsrrc.org.tw
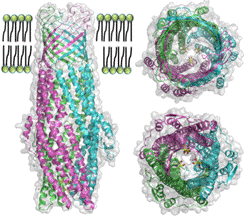
ST50, an outer-membrane component of the multi-drug efflux system from Salmonella enterica serovar Typhi, is an obligatory diagnostic antigen for typhoid fever. ST50 is an excellent and unique diagnostic antigen with 95% specificity and 90% sensitivity and is used in the commercial diagnosis test kit (TYPHIDOTTM). The crystal structure of ST50 at a resolution of 2.98 Å reveals a trimer that forms an α-helical tunnel and a β-barrel transmembrane channel traversing the periplasmic space and outer membrane. Structural investigations suggest significant conformational variations in the extracellular loop regions, especially extracellular loop 2. This is the location of the most plausible antibody-binding domain that could be used to target the design of new antigenic epitopes for the development of better diagnostics or drugs for the treatment of typhoid fever. A molecule of the detergent n-octyl-β-D-glucoside is observed in the D-cage, which comprises three sets of Asp361 and Asp371 residues at the periplasmic entrance. These structural insights suggest a possible substrate transport mechanism in which the substrate first binds at the periplasmic entrance of ST50 and subsequently, via iris-like structural movements to open the periplasmic end, penetrates the periplasmic domain for efflux pumping of molecules, including poisonous metabolites or xenobiotics, for excretion outside the pathogen. The rational designs and strategies of purification and crystallization of membrane protein ST50 for the structural study will be presented and discussed.
|
|
|
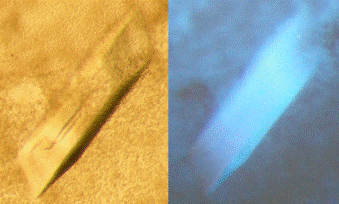
Figure 1. The single crystal of ST50 was illuminated under the white light (left) and the UV light (right). |
Figure 3. The structure of ST50 is viewed from the side (left), top (upper right) and bottom (bottom right). |