Insights into ion selectivity and translocation in the bacterial magnesium channel CorA provided by anomalous diffraction experiments
Natasha Kruglyak1,4, Roland Pfoh1,4, Pedram Mehrabi2,4, Emil F. Pai1,2,3,4
1 Department of Biochemistry, University of Toronto, Toronto, Ontario, Canada
2Department of Medical Biophysics, University of Toronto, Toronto, Ontario, Canada
3 Department of Molecular and Medical Genetics, University of Toronto, Toronto,
Ontario, Canada
4 Campbell Family Cancer Research Institute/Ontario Cancer Institute, Toronto, Ontario, Canada
natasha.kruglyak@mail.utoronto.ca
Magnesium (Mg2+) is the most abundant divalent cation in eukaryotic and prokaryotic cells, having numerous important physiological functions [1]. However, of the four main biological cations, the transport and homeostasis of magnesium remains the least understood. Members of the ubiquitous CorA family of Mg2+ channels contain a canonical Gly-Met-Asn (312GMN314) signature motif at the extracellular mouth of the permeation pathway, which has been proposed to form part of the Mg2+ selectivity filter of these channels [2-4]. In this study, we use anomalous x-ray diffraction to examine the binding at the selectivity filter of two transported substrates of CorA, cobalt (Co2+) and nickel (Ni2+), the non-substrate samarium (Sm3+) as well as the known CorA inhibitor cobalthexammine, which is also an inert structural analogue of hexahydrated Mg2+[5]. Our results indicate that while Co2+ and Ni2+ are able to bind at the 312GMN314 motif coordinating with G312 and N314, Sm3+ is excluded from the selectivity filter. Cobalthexammine is also able to bind at the mouth of the CorA pore, but at a position slightly peripheral to the 312GMN314 motif, suggesting that CorA allows binding of a hexahydrated Mg2+, but only in a position that does not allow further penetration into the selectivity filter, explaining the inhibitory effect of cobalthexammine. Moreover, we suggest that our results strongly support a knock-on mechanism for ion transduction through the CorA pore, where a Mg2+ bound at G312 and N314 is pushed down the permeation pathway by an incoming Mg2+ bound at N314 (Figure 1).
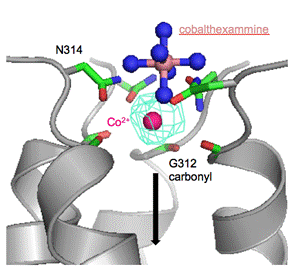
Figure 1. Co2+ and cobalthexammine binding at the GMN motif of CorA. The position of the cobalthexammine is superimposed onto the structure obtained with Co2+. Only four protomers and the anomalous density for Co2+ (cyan mesh) are shown for clarity. The arrow indicates the direction of ion translocation.
1. A. Hartwig. Mutat. Res., 475, (2001), 113.
2. O. Dalmas, W. Sandtner, D. Medovoy, L. Frezza, F. Bezanilla, E. Perozo. PNAS, 111, (2014), 3002.
3. A. Guskov, N. Nordin, A. Reynaud, H. Engman, A. K. Lundback, A. J. Jong, T. Cornvik, T. Phua, S. Eshaghi. PNAS, 109, (2012), 18459.
4. R. Pfoh, A. Li, N. Chakrabarti, J. Payandeh, R. Pomes, E. F. Pai. PNAS, 109, (2012), 18809.
5. L. M. Kucharski, W. J. Lubbe, M. E. Maguire. JBC, 275, (2000), 16767.