Structure of the C345c domain of murine complement component C4
Trine A. F. Gadeberg, Sofia Mortensen, Gregers R. Andersen
Department of Molecular Biology, Aarhus University, Aarhus, Denmark
trineg@mbg.au.dk
Complement is a central part of the innate immune system. It acts as a danger-sensing system and its activation initiates a proteolytic cascade, resulting in a strong inflammatory response [1]. There are three different complement activating pathways; the classical pathway, the lectin pathway and the alternative pathway. The pathways converge in the formation of the proteolytic C3 and C5 convertases. In the classical and lectin pathway, the convertases contain C4b as the substrate recognising subunit, and C2a as the proteolytic subunit. In the classical pathway, C3b and Bb are found as these respective subunits.
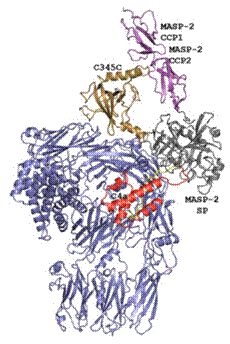
In the lectin and classical pathway, activation is triggered when recognition molecules detects danger signals in the form of carbohydrates or antibodies. MASP-2 and C1s are serine proteases found in complex with the recognition molecules. Upon activation they bind and cleave C4 into C4b and C4a [2, 3]. The binding occurs through the C-terminal C345c domain of C4 interacting with an exosite between two CCP domains of the protease (Fig. 1) [4].
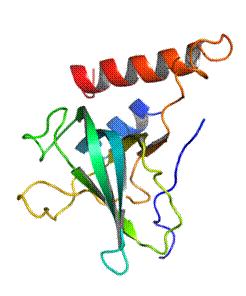
Over-activation of complement is the cause of several diseases [5, 6], and inhibitors of complement activation are interesting to find. It has been found that the C4 C345c domain can act as an inhibitor of the classical and lectin pathways. Future experiments based on this finding would be conducted in a mouse based animal model. Therefore, the structural similarity of the murine C4 C345c domain to its human counterpart is of interest. Here, I present the crystal structure of the murine C4 C345c domain at 2.1 Å resolution (Fig. 2). The structure reveals a positively charged patch on the C4 C345c domain where it interacts with the CCP exosite of MASP-2.
|
|
|
Figure 1. The crystal structure of the C4-MASP2 complex. C4 is blue expect for C4a (red) and the C345c domain (brown). The MASP2 CCP domains (magenta) interact with the C4 C345c domain. [4] |
Figure 2. The crystal structure of the murine C4 C345c domain in the same orientation as in figure 1. The schematics are colored from blue (N terminus) to red (C terminus). |
[1] D. Ricklin, G. Hajishengallis, K. Yang, and J. D. Lambris, “Complement: a key system for immune surveillance and homeostasis.,” Nat. Immunol., vol. 11, no. 9, pp. 785–97, Sep. 2010.
[2] A. J. Perry, L. C. Wijeyewickrema, P. G. Wilmann, M. J. Gunzburg, L. D’Andrea, J. a Irving, S. S. Pang, R. C. Duncan, J. a Wilce, J. C. Whisstock, and R. N. Pike, “A molecular switch governs the interaction between the human complement protease C1s and its substrate, complement C4.,” J. Biol. Chem., vol. 288, no. 22, pp. 15821–9, May 2013.
[3] T. R. Kjaer, S. Thiel, and G. R. Andersen, “Toward a structure-based comprehension of the lectin pathway of complement.,” Mol. Immunol., vol. 56, no. 4, pp. 413–22, Dec. 2013.
[4] R. T. Kidmose, N. S. Laursen, J. Dobo, T. R. Kjaer, S. Sirotkina, L. Yatime, L. Sottrup-Jensen, S. Thiel, P. Gal, and G. R. Andersen, “Structural basis for activation of the complement system by component C4 cleavage,” Proc. Natl. Acad. Sci., vol. 109, no. 38, pp. 15425–15430, Sep. 2012.
[5] G. Bajic, S. E. Degn, S. Thiel, and G. R. Andersen, “Complement activation, regulation, and molecular basis for complement-related diseases,” EMBO J., vol. 34, no. 22, pp. 1–23, 2015.
[6] A. P. Sjöberg, L. A. Trouw, and A. M. Blom, “Complement activation and inhibition: a delicate balance.,” Trends Immunol., vol. 30, no. 2, pp. 83–90, Feb. 2009.