Multiphase Liquid Crystalline Nanoassemblies with Protein Ordering
B. Angelov1, M. Drechsler2, V.M. Garamus3, A. Angelova4
1ELI Beamlines, Fyzikalni ustav, AVCR, 25241 Dolní Břežany, Czech Republic,
2Laboratory for Soft Matter Electron Microscopy, Bayreuth Institute of Macromolecular Research, University of Bayreuth, D-95440 Bayreuth,
3Helmholtz-Zentrum Geesthacht, Centre for Materials and Coastal Research, D-21502 Geesthacht, Germany,
4Institut Galien Paris-Sud, CNRS UMR 8612, Univ. Paris-Sud, Université Paris-Saclay, LabEx LERMIT, 92296 Châtenay-Malabry cedex, France
Email: Borislav.Angelov@eli-beams.eu
Confinement of proteins in nanostructured particles provides a means for substantial concentration of the biomacromolecules and for optimal contacts between the neighbouring molecules towards protein ordering and crystallization. Liquid crystalline nanoparticles of self-assembled lipids and amphiphiles have been known for their advantages as templates for nucleation and growth of nanocrystals. To fulfil the need of 3D biomacromolecular ordering that precedes the protein crystallization, we followed the loading and confinement of a charged therapeutic protein brain-derived neurotrophic factor (BDNF) in PEGylated lipid nanoparticles of liquid crystalline inner organization. The latter were obtained by the method of self-assembly and hydration of lipid mixtures [1-4]. The neurotrophin BDNF plays a key functional role in the differentiation, proliferation, growth, plasticity, and survival of neurons in the central and peripheral nervous systems. This protein exerts its biological activity as a dimmer, although its dimeric form has not been crystallized yet.
Here the dynamic nanoscale organization of lipid/neurotrophic protein assemblies was studied upon progressive loading of the neurotrophin BDNF in lipid membrane particles. Synthetic lipids and human recombinant BDNF of maximal purity were employed for sample preparation. Millisecond time-resolved small-angle X-ray scattering (SAXS) experiments were in situ performed using a rapid-mixing stopped-flow setup coupled to synchrotron SAXS measurements. The neurotrophin BDNF (which lacks conformational flexibility) demonstrated capacity to considerably modify the curvature of the studied flexible lipid membranes. Time-resolved SAXS monitoring of protein molecules entrappment in lipid nanoparticulate containers established the formation and coexistence of double diamond cubic Pn3m (D), gyroid cubic Ia3d (G), and lamellar (L) structures within the investigated nano-objects (Figure 1). The obtained ordered arrays of uploaded BDNF biomacromolecules were able to rapidly alter the membrane curvature at the initial stage of protein loading. Subsequently, the curved packing in the membrane domains was accompanied by protein accumulation in more concentrated areas inside the nanoparticles. This was associated with the formation of cubic and well ordered lamellar domains containing nanoconfined proteins.
The performed cryogenic transmission electron microscopy (Cryo-TEM) study revealed the morphological patterns and shapes associated with the protein ordering. It confirmed the transformation of the inner liquid crystalline lipid structures into organized lipid/protein complexes. Ordered protein patterns emerged as a result of the induction of domains of new ordering upon protein accommodation in the lipid supramolecular assemblies. The obtained structural results evidenced the stages of the protein loading and ordering in lipid nanoparticles and suggested that protein concentrations higher than 4 mg/ml would be required for biomacromolecules assembly into nuclei for 3D protein crystallization.
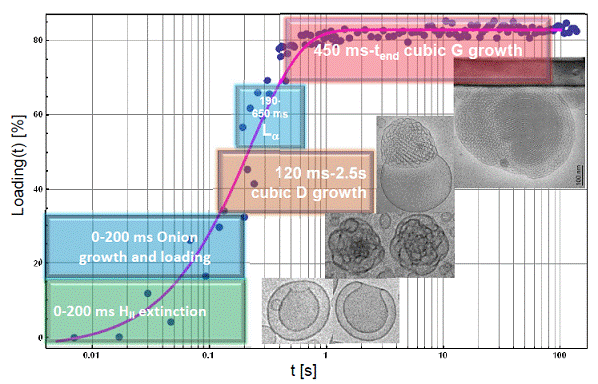
Figure 1. Percentage of protein loading that is associated with the induction of structural transformations of vesicular lipid membranes into growing cubic and mixed liquid crystalline structures with embedded proteins.
1. B. Angelov, A. Angelova, S. K. Filippov, M. Drechsler, P. Štěpánek, S. Lesieur, ACS Nano, 8, (2014), 5216-5226.
2. B. Angelov, A. Angelova, M. Drechsler, V.M. Garamus, R. Mutafchieva, S. Lesieur, Soft Matter, 11, (2015), 3686-3692.
3. B. Angelov, A. Angelova, S. K. Filippov, T. Narayanan, M. Drechsler, P. Štěpánek, P. Couvreur, S. Lesieur, J. Phys. Chem. Lett., 4, (2013) 1959-1964.
4. B. Angelov, A. Angelova, B. Papahadjopoulos-Sternberg, S. Lesieur, , J.-F. Sadoc, M. Ollivon, P. Couvreur, J. Am. Chem. Soc., 128, (2006) 5813 - 5817.