Ionic liquids - water interplay in protein crystallization. From additives to nucleants to...
M. Kowacz1,2, M. Marchel1, L.
Juknaité3, A. Mukhopadhyay3, A. L. Carvalho3,
J. M. S. S. Esperança1, M. J. Romão3
and L. P. N. Rebelo1
1Instituto de Tecnologia Química e Biológica António Xavier, Universidade Nova de Lisboa, Av. da República, 2780-157, Oeiras, Portugal
2Jerzy Haber Institute of Catalysis and Surface Chemistry, Polish Academy of Sciences, Niezapominajek 8, 30-239 Krakow, Poland
3 REQUIMTE/CQFB, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829-516, Caparica, Portugal
nckowacz@cyf-kr.edu.pl; luis.rebelo@itqb.unl.pt
In our study we explore the potential of ionic liquids (ILs) to advance the field of protein crystal growing. ILs are a class of compounds composed of two oppositely charged species, the cation and the anion, that are liquid near room temperature. The number of possible combinations of composing ions is immense and theoretically it is possible to design a given ionic liquid that best suits a specific, desired purpose. However, one first needs to know how the ionic liquid properties relate to that particular purpose, in this case protein crystallization.
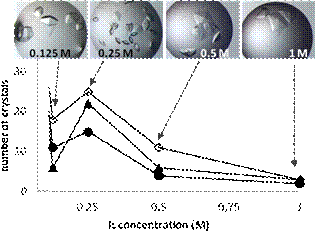
We started from identifying the variables and mechanisms behind the effect of different water soluble ILs used as additives in protein crystallization [1]. It has been recognized that, the same IL ions, which at low ionic strength (IS) conditions bind to the protein surface and screen the charges (promote salting-out and crystal nucleation), at high IS reduce the protein–water interfacial tension by remaining hydrated while attached to the surface (induce salting-in) (Fig. 1). Furthermore, it has to be pronounced that it is necessary to take into account the mutual affinity of the ions and their counterions in order to properly rank the respective (IL) ion pairs as salting-in/out agents.
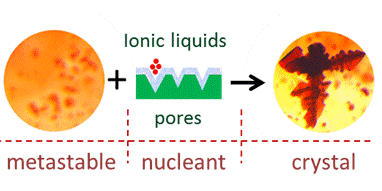
As a next step, we have immobilized hydrophobic ILs at the surface of hydrophilic solid sub-micron particles [2]. This enabled the dispersion in aqueous solution of otherwise water-immiscible ILs and their first use in protein crystallization as heterogeneous nucleants [3] (Fig. 2). It has been recognized that their nucleating potential is determined by the protein-solid affinity based on short range H-bond donating/accepting interactions that can be tuned by changing the ILs structure.
|
Fig. 1 Concentration-dependent salting-in/out behaviour of ILs |
Fig. 2 Schematic action of IL-functionalized nucleants |
Our on-going research is focused on the use of a remote physical trigger – the non-ionizing electromagnetic radiation to induce and control protein crystal growth. We showed the effect of infrared light (IR) on protein crystal nucleation and periodic self-assembly resulting from modulation of protein interfacial water (dipolar medium) by means of IR [4]. Now we want to use ILs as polar and polarisable interfacial solvents prone to respond to electromagnetic stimuli.
1. M. Kowacz, A. Mukhopadhyay, A. L. Carvalho, J.M. S. S. Esperança, M. J. Romão, L. P. N. Rebelo. CrystEngComm., 14, (2012), 4912-4921.
2. M. Kowacz, M. Marchel, J. M. S. S. Esperança, L. P. N. Rebelo. Materials Chem. Phys., 160, (2015), 308-314.
3. M. Kowacz, M. Marchel, L. Juknaite, J. M. S. S Esperança, M. J. Romão, A. L. Carvalho, L. P. N Rebelo. Cryst. Growth Des., 15, (2015), 2994–3003.
4. M. Kowacz, M. Marchel, L. Juknaite, J. M. S. S Esperança, M. J. Romão, A. L. Carvalho, L. P. N Rebelo. J. Crystal Growth, (2016),. DOI: 10.1016/j.jcrysgro.2016.01.003.
Authors acknowledge financial support from Fundação para a Ciência e a Tecnologia, Portugal trough grants SFRH/BPD/63554/2009 and PTDC/BBB-BEP/3058/2012 and a contract under FCT Investigator program. We also thank the National Science Centre (NCN), Poland for providing support trough grant FUGA (2015/16/S/ST4/00465).