From the crystal to the structure thanks to an all in one lanthanide complex
E. Girard1, S. Engilberge1, L. Lassalle1, S. Di Pietro2, F. Riobé2, O. Maury2
1Institut de Biologie Structurale, UMR5075 CEA-CNRS-UJF, EPN Campus, 71 avenue des Martyrs, 38044 Grenoble Cedex 9
2UMR5182 CNRS-ENS Lyon-Université Lyon 1, 46 allée d'Italie, 69364, Lyon cedex
eric.girard@ibs.fr
Since 2000, we developed lanthanide complexes for structure determination of macromolecules, exploiting the high-phasing power of lanthanide elements [1-8]. Recently, we produced a new complex with luminescent properties. Unexpectedly, this complex ([Ln]) showed promising nucleant properties making it the first nucleant, luminescent and phasing agent.
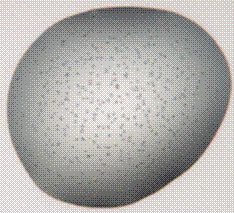
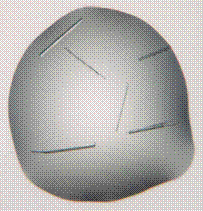
We will present the results of the crystallization properties obtained on 8 proteins including not only commercial ones but also proteins of unknown structures. Potential crystallization hits have been determined using high-throughput crystallization (HTXlab, EMBL, Grenoble) through screening of 576 conventional conditions. We systematically compared the native protein with the one in presence of [Ln]. Results showed that, in most cases, the presence of [Ln] induces a major increase of potential hits and provides new crystallization conditions for all tested proteins. As illustrated in Figure 1, the crystals obtained in presence of [Ln] are better.
|
|
|
Figure 1. Crystallization drops obtained for the same protein without (left) and with the all in one lanthanide complex (right). |
|
The luminescent property of [Ln] allows to facilitate crystal detection in crystallization drops as well as to facilitate crystal centring at synchrotron beamlines. Finally, using crystals obtained with [Ln], the structures of the 8 proteins (including the two unknown ones) have been determined by means of anomalous-based methods. In conclusion, this new all in one lanthanide complex overcomes the two major bottlenecks in protein crystallography: crystallization and phase determination.
1. E. Girard, M. Stelter, P.L. Anelli, J. Vicat, R. Kahn, Acta Crystallogr D Biol Crystallogr , 59, (2003), 118.
2. E. Girard, M. Stelter, J. Vicat, R. Kahn, Acta Crystallogr D Biol Crystallogr, 59, (2003), 1914.
3. R. Talon, L. Nauton, J.-L. Canet, R. Kahn, E. Girard, A. Gautier, Chem. Commun., 48, (2012), 11886.
4. R. Talon, R. Kahn, M.A. Durá, O. Maury, F.M.D. Vellieux, B. Franzetti, E. Girard, Journal of Synchrotron Radiation, 18, (2011), 74.
5. G. Pompidor, O. Maury, J. Vicat, R. Kahn, Acta Crystallogr D Biol Crystallogr., 66, (2010), 762.
6. G. Pompidor, A. D'Aléo, J. Vicat, L. Toupet, N. Giraud, R. Kahn, O. Maury, Angewandte Chemie International Edition, 47, (2008), 3388.
7. E. Dumont, G. Pompidor, A. D'Aléo, J. Vicat, L. Toupet, R. Kahn, E. Girard, O. Maury, N. Giraud, Phys. Chem. Chem. Phys., 15, (2013), 18235.
8. A. D'Aléo, G. Pompidor, B. Elena, J. Vicat, P.L. Baldeck, L. Toupet, R. Kahn, C. Andraud, O. Maury, ChemPhysChem., 8, (2007), 2125.
We acknowledge financial support from the Agence Nationale de la Recherche (ANR-13-BS07-0007-02 Ln23).