Investigations into the binding site promiscuity of MhsT
Caroline Neumann1, Dorota Focht1, Joseph Lyons1, Mathias Quick2, Jonathan Javitch2, Poul Nissen1
1Department of Molecular Biology and Genetics and Dandrite, Aarhus University, Aarhus, Denmark
2Center for Molecular Recognition and Departments of Psychiatry and Pharmacology, Columbia University College of Physicians and Surgeons, New York, USA.
caroline@mbg.au.dk
Neurotransmitter:sodium symporters (NSSs) are secondary transporters, placed on the presynaptic cell of the synapse. They ensure uptake of neurotransmitter molecules from the synaptic cleft, using energy released from the downward movement of Na+ along its electrochemical gradient. Therefore, NSSs have an important role in controlling impulse signaling in neurons and a number of diseases are related to dysfunctions of neurotransmitter transporters. Insight into transporter structure and function has been obtained from structures of bacterial and eukaryotic members.
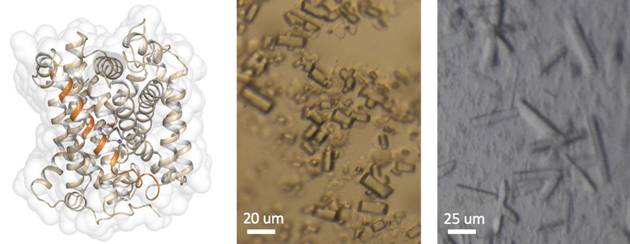
MhsT is a hydrophobic amino acid transporter [1] and a member of the NSS family. The goal of this project is to study the structural basis of the binding site promiscuity of MhsT. Crystal structures of MhsT in complex with L-Phe (2.25 Å) and a tyrosine orthologue L-4-F-Phe (2.26 Å) have been determined from crystals grown using the HiLiDe method [2]. Like the previously determined MhsT+Trp structure [3], the protein is in an occluded inward-facing conformation for the two substrates. This conformation of the protein reflects the state just before the opening of the transporter to the intracellular side, and gives insight into how sodium drives this process [3]. The binding sites, however, while similar have some important differences that mark the bound substrate. Based on these structures, binding site mutations of MhsT, to resemble that of the human neutral amino acid transporters SLC6A18 and SLC6A19, have been generated. Structural and functional investigations of these mutants will provide a better insight into the binding specificity of this transporter.
|
|
Figure 1. On left: The overall structure of MhsT in its occluded-inward facing state, what is indicated by TM5 being unwound in its intracellular part (TM5 is marked as an orange helix). On right: Various crystals of MhsT+Phe obtained using the HiLiDe method.
|
1. Quick, M. & Javitch, J. A. Monitoring the function of membrane transport proteins in detergent-solubilized form. PNAS 104, (2007), 3603
2. Gourdon, P., Lauwring Andersen, J., Langmach Hein, K., Bublitz, M., Panyella Pedersen, B., Liu, X., Yatime, L., Nyblom, M., Terndrup Nielsen, T., Olesen, C., Vuust Møller, J., Nissen, P. and Preben Morth, J. HiLiDe, Systematic approach to membrane protein crystallization in lipid and detergent. Cryst. Growth Des 11, (2011) 2098
3. Malinauskaite, L., Quick, M., Reinhard, L., Lyons, J. A., Yano, H., Javitch, J.A, Nissen, P. A mechanism for intracellular release of Na+ by neurotransmitter/sodium symporters. NSMB 21, (2014), 1006