Microfluidic platform for optimisation of crystallisation conditions
C. Gerard1, S. Zhang1, A. Ikni1, G. Ferry2, L.M. Vuillard2, J. Boutin2, N. Ferte1, R. Grossier1, N. Candoni1, S. Veesler1
1CINaM - CNRS, Aix-Marseille Université, Campus de Luminy, Case 913, F-13288 Marseille Cedex 09
2Institut de Recherches SERVIER, 125, chemin de ronde, F-78290 Croissy-sur-Seine.
gerard@cinam.univ-mrs.fr
Over the past few decades, the science of crystallisation has attracted vast interest in industrial and academic applications. In order to get suitable quality crystals, the crystallisation process is monitored and controlled through the screening of favourable crystallisation conditions and subsequent optimising of crystal growth by developing a specific kinetic path in the phase diagram. In recent years, crystallisation of proteins or pharmaceuticals has been improved thanks to high-throughput techniques, offering multiple operations such as mixing, analysis, separation. In one hand, high-throughput robots rely on automated liquid-handling techniques integrated with dispensers and fluidic circuits[1]. In return they generally require high budget. In the other hand, microfluidic techniques controlling and manipulating flows at even lower scales (micro, nano to picoliter) use miniaturised devices called lab on chip [2, 3]. However, these devices are not very accessible to non-specialists of microfluidics due to their complexity.
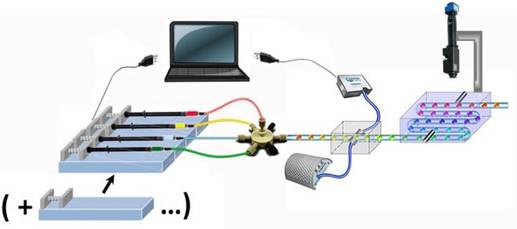
Figure 1. Scheme of the home-made microfluidic platform: (1) syringe pump, (2) 7-entry junction, (3) on-line UV module (4) tubing-holder for thermostatting and observation and XYZ-motorized camera.
We develop a microfluidic platform accessible to non-specialists of microfluidics and applicable to soluble and membrane proteins in aqueous and organic solvents. It is based on the notion of modular microfluidics [4], with discrete components that can be easily assembled, replaced or removed in a plug-and-play mode. It includes modular sub-functions such as droplet generation and storage, on-line UV characterisation and observation (Fig.1). Thus, compared to traditional methods, it offers advantages with respect to system cost, planning, and maintenance.
We used this platform for the optimisation of crystallisation conditions of a protein. Therefore, the chemical composition, size and frequency of microdroplets were measured on-line by UV spectrophotometry. An initial condition was deduced from a rapid screening. Then we varied the concentrations of protein and crystallisation agent in hundreds droplets of identical sizes and we observed their influence on the crystallisation until we obtained 1 crystal per droplet. We expect this platform will be easily incorporated into any laboratory, such as chemical, pharmaceutical, biological.
1. R.C. Stevens, Current Opinion in Structural Biology, 10 (2000) 558-563.
2. C.L. Hansen, E. Skordalakes, J.M. Berger, S.R. Quake, Proceedings of the National Academy of Sciences, 99 (2002) 16531-16536.
3. L. Li, D. Mustafi, Q. Fu, V. Tereshko, D.L. Chen, J.D. Tice, R.F. Ismagilov, Proceedings of the National Academy of Sciences, 103 (2006) 19243-19248.
4. S. Zhang, N. Ferté, N. Candoni, S. Veesler, Organic Process Research & Development, 19 (2015) 1837-1841.