A new design of plate geometry for efficient protein crystallization screening
D. C. Yin*, C. Y. Zhang, R. Q. Chen, C. Dong, R. B. Zhou, Q. D. Cheng
School of Life Sciences, Northwestern Polytechnical University, Xi’an 710072, Shaanxi, PR China
yindc@nwpu.edu.cn
We propose in this report a new design of geometry for protein crystallization plates. Each plate comprises 96 units corresponding to the 96 conditions of commercial crystallization screening kits. Each unit consists of 4 wells in which four different volume ratios of protein solution to precipitant solution can be set up. Based on the geometry we manufactured two types of crystallization plates: 1) Microbatch plate (M plate): the 96 units are separately sealed but the 4 wells in each unit are sealed to share the same common space; 2) Cross-diffusion microbatch1 plate (CDM plate): all 96´4 wells are sealed to share the same common space, so that all volatile components in the droplets can freely diffuse in the common space. Figure 1 shows schematically the geometry of these two crystallization plates.
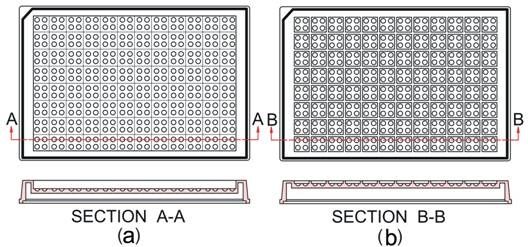
Figure 1. Two types of crystallization plates used in this study. (a) CDM plate; (b) M plate1.
The crystallization plates exhibit strong flexibility to adopt different crystallization screening strategies. A very promising method is to vary the volume ratio of protein solution to precipitant solution among the 4 wells. Hence, 4 different initial concentrations can be simultaneously used for each precipitant solution, so that more chances of obtaining crystals may be achieved.
|
|
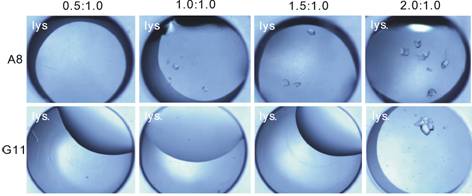
Figure 2. Crystallization examples at different initial volume ratios of protein solution to precipitant solution2. |
Figure 2 shows some examples that, different initial volume ratios can provide more chances to obtain crystals: when at one initial concentration, there may be no crystals at all (like in those clear drops), but at other initial concentrations using the same precipitant, crystals may appear (like in those drops with crystals).
Figure 3 shows the comparisons of crystallization results using CDM and M plates as compared against the traditional sitting drop vapour diffusion (SDVD) plate. It can be seen that both CDM and M plates are powerful to increase the efficiency to crystallize proteins.
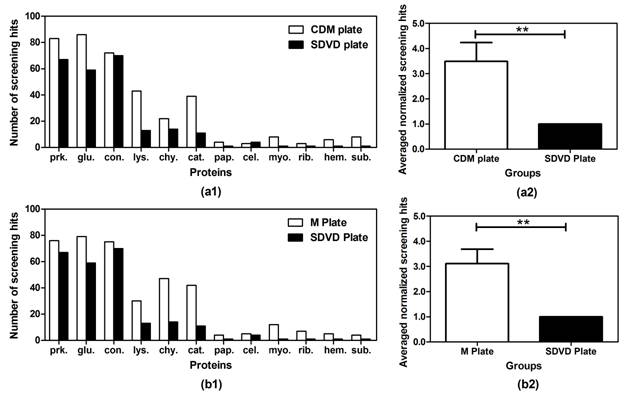
Figure 3. Comparisons of the number of crystallization screening hits using different crystallization plates (SDVD Plate, M Plate, and CDM Plate)2. The number of crystallization screening hits for the M and CDM plates is without redundancy, i.e., hits in one unit count as one hit. (a1) Comparisons of the number of screening hits between CDM and SDVD Plates. (a2) The averaged normalized screening hits on the CDM plate compared to the SDVD Plate, **P<0.01, error bar: mean ± SEM, n =12. (b1) Comparison of the number of screening hits between M and SDVD Plates. (b2) The averaged normalized screening hits on the M plate compared to the SDVD Plate, **P<0.01, error bar: mean ± SEM, n =12.
Apart from varying the volume ratios of protein solution to precipitant solution, another strategy is to vary the pH level in the droplet. By adding an extra buffer at different pH to the wells separately, we may adjust the final pH in the crystallization droplet, thus creating different crystallization conditions simultaneously.
1. R. Q. Chen, D. C. Yin, Y. M. Liu, Q. Q. Lu, J. He,Y. Liu, Acta Cryst. D70, (2014), 647.
2. C. Dong, C. Y. Zhang, Y. Y. Liu, R. B. Zhou, Q. D. Cheng, D. C. Yin, Cryst. Growth Des. DOI: 10.1021/acs.cgd.5b00702.
This work was supported by National Natural Science Foundation of China (Grant No. 31170816).