Biophysical and structural characterization of dipeptidyl peptidase III from Porphyromonas gingivalis
|
|
|
|
|
|
|
|
Hromic A.1, Kumar P.1, Das K.M.P.1, Madl T.4, Jajcanin-Jozic N.2, Wallner S.3, Abramic M.2, Oberer M.1, and Gruber K.1
1University of Graz, Austria,
2Institute Rudjer Boskovic Zagreb, Croatia,
3Graz University of Technology, Austria,
4Medical University Graz, Austria
Porphyromonas gingivalis is a gram-negative human pathogenic bacterium. It is found in the oral cavity and is able to break through human gingival fibroblasts causing difficult and painful diseases like gingivitis and periodontitis [1]. It also contains a group of enzymes which belong to the dipeptidyl peptidase III (DPP III) family. This group of enzymes, also known as enkephalinase B, is an enkephalin-degrading enzyme that cleaves dipeptides sequentially from the N-termini of its substrates. All DPPs III described thus far contain the unique zinc binding motif HEXXGH characteristic for the metallopeptidase family M49. DPP III play important role in the mammalian pain modulatory system. This is supported by the finding that low levels of DPP III activity were detected in the cerebrospinal fluid of individuals suffering from acute pain [2].
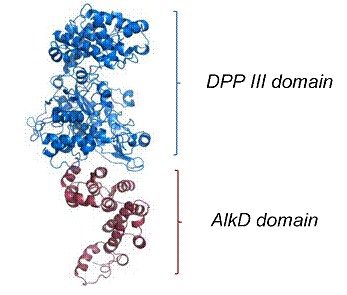
The function of the DPP III homolog from Porphyromonas gingivalis is still unknown, but it could be involved in pathogenicity. We aim at determining potential substrates of this enzyme as well as its three-dimensional X-ray structures in order to obtain information about its potential function. Here, we represent and discuss ITC and SAXS data together with predicted structural model. We also present findings about potential inhibitor of the enzyme using conformational changes calculated from SAXS measurements. Additionally, we briefly investigate C-terminal end of the enzyme since it possesses structural similarities with alkylpurin DNA glycosylase AlkD from B.cereus.
[1] Mysak, J et al., (2014): Porphyromonas gingivalis: Major Periodontopathic Pathogen Journal of Immunology Research, Volume 2014, Article ID 476068, 8 pages
[2] Bezerra, G et al., (2012): Entropy-driven binding of opioid peptides induces a large domain motion in human dipeptidyl peptidase III, PNAS, vol. 109 no. 17, 6525–6530, doi: 10.1073/pnas.1118005109