Towards understanding and inhibiting the hook-into-groove interaction of the herpesviral nuclear egress complex
J. Schweininger, M. Kriegel, Y. A. Muller
Division of Biotechnology, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany
johannes.schweininger@fau.de
Herpesviruses are ubiquitous human pathogens. After genome replication and capsid assembly, transport of the capsids through the nuclear membrane and into the cytoplasm is mediated by the conserved nuclear egress complex (NEC) with its signature hook-into-groove interaction (Fig. 1).
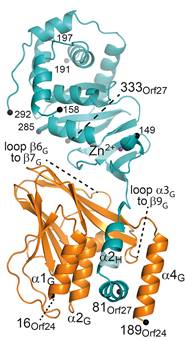
We have recently solved the crystal structure of the varicella-zoster virus (VZV) NEC composed of the proteins Orf24 and Orf27 (PDB: 7PAB, Fig. 1), which closely resembles the NECs of herpes simplex virus (HSV-1), pseudorabies virus (PRV), human cytomegalovirus (HCMV) and Epstein-Barr virus (EBV) [1]. Computational and biophysical characterisation of the interaction revealed distinct differences between α- (VZV), β- (HCMV) and γ-herpesviruses (EBV).
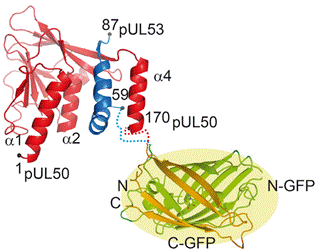
The hook-into-groove interaction presents itself as a promising target to combat herpesvirus infections. To investigate this, we have adopted a fluorescence-based screen utilising Split-superpositive GFP (Sp-spGFP) to identify short peptides from large libraries that could constitute potential inhibitors of the hook-into-groove interaction [2]. To that end, the respective halves of Sp-spGFP were fused to 15- to 22-residue libraries with six randomised positions as well as pUL50 and co-expressed in E. coli (Fig. 2). Cells with the highest fluorescence were sorted and enriched multiple times. Binder sequences were obtained using next generation sequencing and are currently being analysed in vitro.
|
|
|
Figure 1. VZV NEC composed of Orf24 and Orf27 with hook-into-groove interaction. |
Figure 2. Schematic representation of pUL50 and the pUL53 hook (PDB: 6T3X) fused to GFP (PDB: 1EMA). |