Architecture and functional dynamics of a large, virulence-associated DNA helicase from enterohaemorrhagic Escherichia coli
Lena M. Grass1, Chao Chen1, Eva Absmeier1, Tarek Hilal2, Bernhard Loll1, Markus C. Wahl1,3
1Freie Universität Berlin, Institute of Chemistry and Biochemistry, Laboratory of Structural Biochemistry, Takustraße 6, D-14195 Berlin, Germany
2Freie Universität Berlin, Institute of Chemistry and Biochemistry, Research Center of Electron Microscopy and Core Facility BioSupraMol, Fabeckstr. 36a, D-14195 Berlin, Germany
3Helmholtz-Zentrum Berlin für Materialien und Energie, Macromolecular Crystallography, Albert-Einstein-Straße 15, D-12489 Berlin, Germany
L.Grass@fu-berlin.de
Production of flagella in enterohaemorrhagic Escherichia coli (EHEC) supports EHEC virulence and requires the EHEC-specific nucleic acid-dependent NTPase, Z5898.1 Z5898 has been suggested to represent a separate clade of DExH-box RNA helicases in EHEC and related bacteria.2 Here, we find that Z5898 actually exhibits efficient 3’-to-5’ directional DNA helicase activity, but lacks RNA helicase activity. Cryogenic electron microscopy-based structural analyses of Z5898 in complex with DNA and an ATP analog revealed core helicase domains as also found in the antibiotics resistance DNA helicase, MrfA.3 Although MfrA-like domains in Z5898 are interspersed with and expanded by a dimerization domain, a duplex binding domain, three zinc-binding domains and two phospholipase D-like domains, they assemble a MrfA-like core structure in 3D (Fig. 1). The dimerization motif can flexibly connect two Z5898 molecules, and the oligomeric state depends on the chemical environment and the DNA-binding mode. Comparison of Z5898 in various structural states revealed a dynamic interplay between several domains, structural motifs and bound DNA. Structure-guided mutagenesis in conjunction with functional assays pinpointed a DNA-binding rope, an ATPase activation clamp and a DNA gate as key molecular tools of the helicase. Our results reveal that Z5898-like nucleic acid-dependent NTPases have to be re-classified as DNA helicases and hint at possible strategies to interfere with the virulence-related activities of these enzymes.
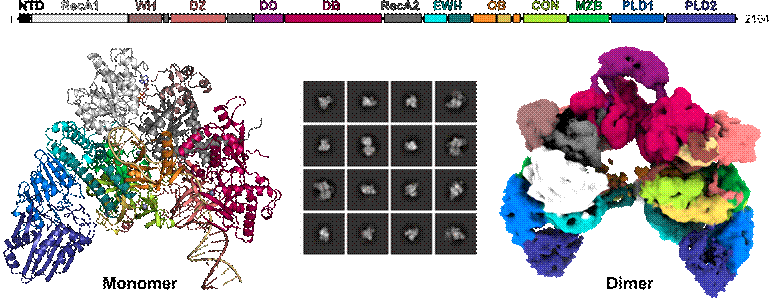
Figure 1: Top, domain composition of the EHEC DEAH/RHA-type helicase Z5898. Left, cartoon representation of monomeric Z5898 in complex with ATPүS and DNA. Middle, 2D class averages of picked particle images during cryoEM analysis. Right, cryoEM density map of dimeric Z5898 in complex with ATPүS and DNA.