High-resolution structure analysis of a small plant biocatalyst at sub 2 Å resolution by Cryo-EM
Carl Helmer1, Nicole Dimos1, Tarek Hilal1,2, Markus Wahl1, Bernhard Loll1
1 Institute of Chemistry and Biochemistry, Department of Biology, Chemistry, Pharmacy, Laboratory of Structural Biochemistry, Freie Universität Berlin, Takustr. 6, 14195 Berlin, Germany
2 Department of Chemistry and Biochemistry, Research Center of Electron Microscopy and Core Facility BioSupraMol, Freie Universität Berlin, Fabeckstr. 36A, 14195 Berlin, Germany
carl.helmer@fu-berlin.de
More than ever a sustainable and efficient alternative to traditional chemical synthesis based on the deployment of mineral oil is prerequisite in the chemical and pharmaceutical industry. Enzyme catalysis combines both demands, as biocatalysts function under mild reaction conditions and possess excellent selectivity. Particularly plants provide biosynthetic enzymes with exquisite specificity that facilitate complex reactions, like the formation of intricate terpene carbon skeletons. Single particle analysis of the structure of such small proteins has a tremendous potential to increase the rational element of protein engineering and spark the capability of e.g. Cryo-EM for structure solution [1]. Cryo-EM already revealed a stunning success in structure solution of large molecular machines, however a major challenge for this technique are small proteins, such as a large amount of biorelevant catalysts are. Small protein complexes have been only reported to a maximum resolution of 3.0 Å yet [2]. Hence, single particle analysis by Cryo‑EM towards the rapid elucidation of small protein structures has a vast potential to increase the rational element of structural biochemistry and protein engineering in the future. Here, we present the highest resolution achieved by Cryo-EM so far. The structure of a ~120 kDa plant borneol dehydrogenase with outstanding volume clearly below 2 Å resolution (Figure 1) [3]. Considering that structures of homomultimeric plant enzymes are highly under-represented to date and given the molecular weight of the tetrameric complex, we were able to push the boundaries of this rapidly evolving method drastically. Hence, Cryo-EM is a valuable tool to achieve fast and high-resolution structure determination for enzymes that proved difficult to crystallize.
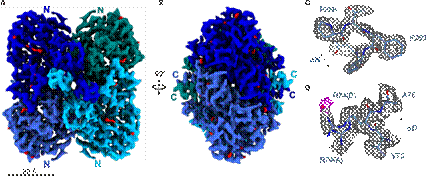
Figure 1: SrBDH1 Cryo-EM structure at 1.88Å. (A) Tetrameric assembly of SrBDH1. Density of each of the four protomers is shown in different blue tones. (B) Rotation by 90°, same color-coding as in A. (C) Zoom on C-terminal end of αH with well-depicted density-hole for aromatic residue F260. (D) Residue R74 in double conformation and water molecule in 2.5 Å distance to guanidinium function. [3]