Crystal Structure and Microstructure of Nanosize Li-La Ferrites
M.
Vučinić-Vasić1), A. S. Nikolić2), J. Blanusa3),
A. Kremenović4), S. Rakić5),
A. Kapor5) and B. Antić2)
1) Faculty of Technical Sciences, University of Novi Sad, Trg D. Obradovica 6, 21000 Novi Sad, Serbia and Montenegro
2) Faculty of Chemistry, Department for Inorganic Chemistry, University of Belgrade, P. O. Box 158, 11001 Belgrade, Serbia and Montenegro
3) Institute of Nuclear Sciences ”Vinca”, Laboratory of Solid State Physics, P. O. Box 522, 11001 Belgrade, Serbia and Montenegro
4)
Faculty of Mining and Geology, Laboratory for Crystallography, University of
Belgrade, P. O. Box 162, 11001 Belgrade, Serbia and Montenegro
5) Institute of Physics, University of Novi Sad, Trg D. Obradovica 4, 21000 Novi Sad, Serbia and Montenegro
Ferrites with spinel structure have a general formula AB2X4 with cations occupying two sites, tetrahedral 8a (A site) and octahedral 16d (B site) in the space group (S.G.) Fd-3m. Some spinels possess ordered structure with possible cation ordering at tetrahedral or/and octahedral sites, where this ordering causes formation of two new sublattices in one (or both) A/B sublattice [1]. Nanosize ferrites are important magnetic materials both for application in technology and in basic researches, as convenient systems for studies of nanomagnetism. It is well known that properties of nanomaterials strongly depend on the preparation conditions. One of the most interesting methods for preparation of ultrafine powders is from complex compounds as precursors. Most metals make complexes with b-diketonato ligands: acetylacetone (AA), benzoylacetone (BA), dibenzoyl-methane (DBM), trifluoracetone (TFA), etc. [2]. Prepared complex compounds have relatively low temperatures of thermal decomposition, giving ultrafine powder as a product [2]. Nanosize Li-La ferrite sample was synthesised by thermal decomposition of appropriate mixture of complex compounds with acetylacetone – (2,4 pentadione) ligands ([M(AA)x]; M=Li, La and Fe) in molar ratio 1(Li):0.1(La):4.9(Fe) at 500 °C. crystal structures of obtained phases were determined by the Rietveld method. Magnetic properties of ferrites are sensitive on the microstructure. Consequently, one of the aims was microstructure determination.
X-ray diffraction data for ferrite sample was collected at a Philips PW 1830 (CuKa radiations) difractometer. The scanning range was 10-110° in 2q, with the step of 0.02° and scanning time of 10s per step. The element analysis (microanalysis) was performed by inductively coupled plasma optical emission spectroscopy (Spectroflame ICP, 2.5KW, 27MHz). Scanning electron microscope (SEM) measurement on the sample was performed on JEOL JSM6460LV. The sample has been sputtered under vacuum with gold metal.
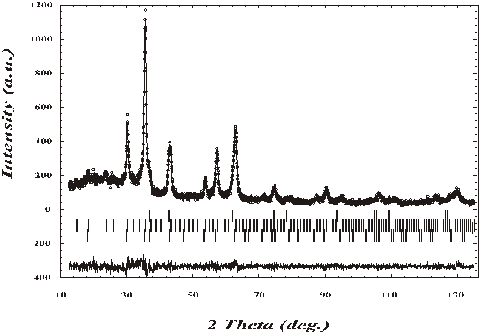
Figure 1.
X-ray diffraction patterns of Li-La ferrite sample.
Dots denote experimental values, line represents calculated values. The
difference between experimental and calculated values is given in the bottom.
The vertical bars indicate reflection positions for all three phases
separately.
The crystal
structure of as-prepared sample was checked by X-ray diffraction. Previously investigation of Li ferrites prepared by the same method
has shown that this method leads to multiphase content [3]. ICP element analysis data show La contents
larger than in expected phase Li0.5La0.05Fe2. 45O4. To choose an appropriate structure model, we have considered the
literature data on the formation of multiphase samples of the Li-ferrites
together with the ICP results. Crystal structure
refinement for the Li-La ferrite sample, based on three-phase model (Li0.5LaxFe2.5-xO4+ LaxFe3O4+ LiFeO2) has given the best results. The refinement was performed with
the Fullprof computer program [4]. The chemical
composition of the present phases was determined by the refinement of the
occupation numbers. The obtained results show good agreement with the ICP
element analysis data: 63±3 % of Fe (66±1 % from
ICP), 2.2±0.2 % Li (1.73±0.05 % from ICP) and 4.9±0.3 % of La (4.86±0.08 % from
ICP). The
partial results of the Rietveld refinement listed in Table 1. Diffraction patterns for three phases sample
is shown in the Figure 1.
In the crystal structure of Li0.5La0.08Fe2. 42O4 Li+ and Fe3+ ions dominantly occupy 4b sites and 12d sites, respectively, while La3+ ions preferently occupy octahedral 12d site. However, beside lithium in 4b sites a significant quantity of iron is present, and vice versa for 12d sites. Phase La0.14Fe3O4 was produced by lanthanum insertion into Fe3O4. The mechanism of lithium insertion in ferrite with spinel structure was suggested in reference [5].
Table 1. Refined crystal parameters and corresponding agreement factors
|
Phase |
Li0.5La0.08Fe2. 42O4 (S.G. P4332) |
La0.14Fe3O4 (S.G. Fd-3m) |
LiFeO2 (S.G. Fm-3m) |
|
Percentage
of phase (%) |
69
(1) |
16
(1) |
15
(1) |
|
Lattice
parameter (Å) |
8.3445 (3) |
8.403 (1) |
4.2291 (8) |
|
RB(%) |
3.91 |
3.94 |
2.80 |
|
χ2 |
1.25 |
1.25 |
1.25 |
Microstructure parameters (crystallite size of ~ 11 nm and strain of ~ 1.2 x 103) were determined by the Rietveld refinement of the TCH-pV parameters. To simplify model for the profile Rietveld refinement it was assumed that size-strain X-ray line broadening is the same for all three phases. The particle size determined from SEM micrograph (Figure 2) is ~ 50-60 nm. Thus, the particles are composed of few crystallites.
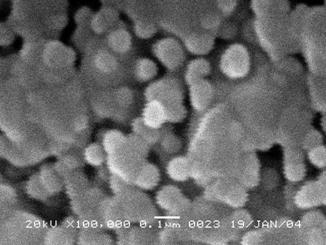
Figure 2. SEM micrograph of particles for Li-La ferrite sample
1. C. Hass: J. Phys. Chem. Solids 26, 125 (1965)
2.
A.S. Nikolić,
N.Cvetković, S. Djuric, J. Puzovic and M. B. Pavlovic: Mat. Sci. Forum 199,
282 (1998)
3.
M.
Vučinić-Vasić, B. Antić, J. Blanusa, S. Rakić, A. Kremenović, A. S. Nikolić and
A. Kapor, submitted to Applied Physics A
4. J. Rodriguez-Carvajal, FullProf computer program, 1998, ftp://charybde.saclay.cea.fr/pub/divers/fullprof.98/windows/winfp98.zip.
5.
L. A. de Picciotto and M.
M. Thackeray: Mat. Res. Bull. 21,
583 (1986)