Diffraction studies of the atomic vibrations of bulk and surface atoms in the reciprocal and real spaces of nanocrystalline SiC
S. Stel'makha ,
a
b
c Swiss-Norwegian Beamlines at ESRF,
d Institut de Cristallographie, Université
de Lausanne, CH-1015 Lausanne, Switzerland
eLos Alamos Neutron Science Center, Los Alamos, NM 87545, USA
f BAE Systems, NASA,
To describe and evaluate the vibrational properties of nanoparticles it is necessary to distinguish between the surface and the core of the particles. Theoretical calculations show that vibrational density of states of the inner atoms of nanograins is similar to bulk material but shifted to higher energies [1, 2] which can be explained by the fact that the grain core is stressed (hardened) due to the presence of internal pressure. Theoretical calculations also show that there is a difference between vibrational properties of a crystal lattice of the grain interior in isolated particles and in a dense (sintered) nanocrystalline material. This is probably due to a coupling of the modes inside the grains via the grain boundaries in dense nanocrystalline bodies [1].
We examined strains present in the surface
shell based on examination of diamond [3] and SiC nanocrystals [4] in
reciprocal (Bragg-type scattering) and real (PDF analysis) space analysis of
neutron diffraction data. Recently we examined the atomic thermal motions in
nanocrystalline SiC based on the assumption of a simple Einstein model for
uncorrelated atomic motions [5].
According to this model, the Bragg intensity is attenuated as a function
of scattering angle by the Debye-Waller factor.
Based on this assumption overall temperature factors were
determined from the
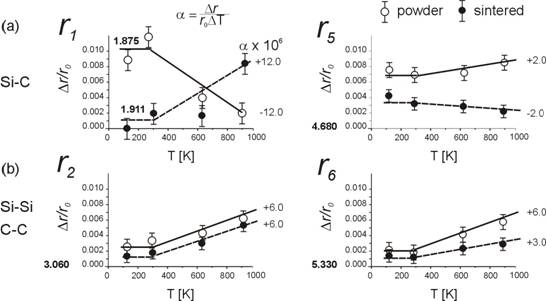
Fig.1 Change of inter-atomic
distances Si-C and Si-Si (C-C) with temperature determined from the PDF
analysis of X-ray diffractograms (BM06 line at ESRF, Qmax = 12
Å-1). Note: The thermal expansion coefficients
determined from the positions of the Bragg reflections for powder and sintered
SiC have the same value (about 4.5 10-6 K-1 [5]).
Using the BT values measured at different temperatures we estimated the Debye temperatures of loose powders and the same powders sintered under high-pressure high-temperature conditions [5]. We found that for a given SiC nano-powder the Debye temperature increased by a few hundred degrees after its sintering. From that we conclude that there is a "hardening" of the material accompanying the transformation of the free surface into the grain boundaries. The above results were obtained for one size (~11 nm) SiC nanocrytals where differences between the atomic motions are related to the surrounding of the crystallites [5].
The analysis of Bragg scattering data yields information on the overall lattice expansion and overall atomic motions without regard to whether they are correlated or not. To extract information on the correlated atomic motions alone the total scattered intensity has to be examined using the atomic Pair Distribution Function analysis [7] which, in principle, probes individual interatomic distances rather than the periodicity of the crystal lattice. Using the diffraction data measured at ESRF in the Q-range of 12-13 Å-1, we performed preliminary PDF analysis showing that there is a clear indication of a difference in elongation between the shortest (Si-Si, C-C) and Si-C interatomic distances, Fig.1.
We showed that thermal expansion and thermal
vibrations in nanocrystalline SiC are dependent on the processing conditions of
the material. The observed differences
between our samples can be understood and interpreted considering that the
structure of the surface is strongly influenced by the environment of the
crystallites. The thermal properties of
the surface have a very strong effect on the overall thermal properties of the
materials.
1. R. Meyer, L.J. Levis, S. Prakash, and P. Entel, Phys. Rev. B68, 104303 (2003).
2. A. Kara and T.S. Rahman, Phys. Rev. Lett. 81, 1453 (1998).
3. B.
Palosz, E. Grzanka, S. Gierlotka, S. Stel'makh, R. Pielaszek, U. Bismayer, J.
Neuefeind, H.-P. Weber, Th. Proffen, R. Von Dreele, and
4. B.
Palosz, E. Grzanka, S. Stel'makh, S. Gierlotka, R. Pielaszek, U. Bismayer,
H.-P. Weber, Th. Proffen, and W.
Palosz, Solid State Phenomena, Eds. W. Lojkowski and J.R. Blizzard, Scitec Publications, 94, (2003).
5. S.
Stel'makh, S. Gierlotka, E. Grzanka, H.-P. Weber, and B. Palosz, , J. Alloys and Compounds (Proc. E-MRS Meeting
2003), in print.
6. M.F.C. Ladd, and R.A. Palmer, Structure Determination by X-Ray Crystallography (Plenum Press, New York, 1994).
7. Il-Kyoung Jeong, T. Proffen, F. Mohiuddin-Jacobs, and S.J.L. Bilinge, J. Phys. Chem A 103, 921 (1999).