The Polymorphs of n-Octadecylammonium Chloride - a Variable-Temperature Powder Diffraction Study
G.J. Krugera, M. Rademeyera and D.G. Billingb,
a Rand Afrikaans University, Box 524, Auckland Park 2006, South Africa, b University of the Witwatersrand, Private Bag 3, Wits 2050, South Africa.
Keywords: alkylammonium halides; polymorphism; X-ray diffraction; thermal analysis.
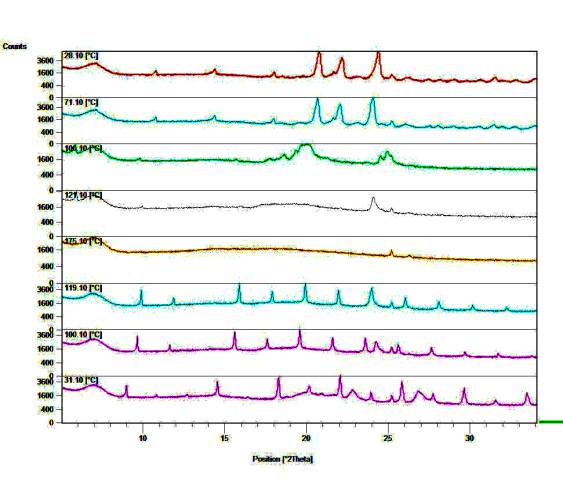
n-Alkylammonium halides (n-CnH2n+1NH3+X-,) are used as surfactants, lubricants, and as models for biological membranes. A spectrum of intermolecular interactions are present in the crystals, ranging from van der Waals interactions between the hydrocarbon chains to ionic and hydrogen bonding interactions in the polar layer. The molecules pack in alternating polar and hydrocarbon layers. Long-chain n-alkylammonium halides exhibit polymorphism at room temperature and a complex sequence of solid-solid phase transitions at higher temperatures. The polymorphs of the n-alkylammonium chlorides, with alkyl chain lengths up to 18 carbon atoms, were studied by powder and single crystal X-ray diffraction and by thermal analysis techniques. All members of this series exhibit polymorphism. The crystal forms can be classified according to their long spacings as determined by powder diffraction. The i form has the smallest long spacing and the k form the largest. The crystal structures of the i and k forms of n-octadecylammonium chloride, C18H37NH3+Cl-, were determined by powder and single crystal X-ray diffraction methods. In the i form the molecular chains are in the all-trans conformation and interdigitated whilst in the k form the chains are non-interdigitated and not completely extended, exhibiting a kink between the second and third carbon atoms. Hydrogen bonding interactions are present between the ammonium groups and chloride anions in the ionic layers. The transition sequences between these forms and the high temperature phases could be determined by thermal analysis (DSC) and a phase sequence diagram constructed. Variable-temperature powder diffraction studies confirmed the transition sequences and proved that the e form consistently crystallizes from the melt.