Powder diffraction and molecular modeling in nano-materials design
P. Čapková1, M. Pospíšil1, Z. Weiss2, J. Šimoník3, H. Schenk4
1Charles University, Faculty of Mathematics and Physics, Ke Karlovu 3, 121 16 Praha 2, Czech Republic.
2 Institute of Materials Chemistry, Technical University Ostrava, 70833 Ostrava-Poruba, Czech Republic.
3 Tomas Bata University, Náměstí T. G. Masaryka 275, 76272 Zlín, Czech Republic.
4 University Amsterdam, Nieuwe Achtergracht 166, 1018 WV Amsterdam, The Netherlands;
Investigation of structure-properties relationship in nanocomposites is very often obstructed with the structural disorder and low quality of powder diffraction data. In that case the molecular modeling using empirical force field represents very powerful tool of structure analysis, providing that the strategy of modeling is based on experiment (X-ray powder diffraction, IR/Raman spectroscopy, NMR….). Using combination modeling and experiment we analyzed structures of nanocomposite applicable in two fields:
· Photofunction units
· Polymer-clay nanocomposites
Technology in both cases is based on intercalation of layer silicates.
Intercalation of organic dyes into layered silicates is one method of producing ordered organic-inorganic hybrid materials with interesting photo-functions [1-3]. Especially the smectite group represents very convenient host structure for the intercalation of organic dyes for two reasons: (i) first of all smectites are transparent in the visible wavelength region and (ii) in addition smectites as host structures provide features attractive for intercalation (swelling behavior, ion-exchange properties). Various dye-clay systems offer an interesting area of material research targeted to the development of new photo-function units. The optical properties of these dyes are environmentally sensitive and depend on the arrangement and configuration of the dye molecules. Anchoring the dye molecule on the silicate layer or other host structure offers the tunning of the emitted wavelength. Rhodamines, which have a high absorption coefficient and the fluorescence yield, are convenient dyes for collecting and utilizing photoenergy. Structure analysis of montmorillonite intercalated with Rhodamine B cations - [RhB]+ was carried out using the combination of modeling, X-ray powder diffraction and IR spectroscopy. The results of structure analysis explained the fluorescence behavior (i.e. profile and position of bands in the emission spectrum) of RhB intercalated montmorillonite in dependence on Rhodamine B concentration and on the way of preparation [4,5]. Figure 1 shows two examples of Rh-montmorillonite intercalated structures prepared under different conditions (different concentration of guest molecules in the intercalation solution).
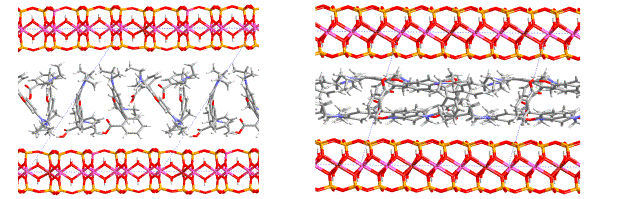

Figure 1
Polymer-clay nano-comoposites exhibit thermal and mechanical properties which make this material attractive in construction business in building industry and machine building. The aim of intercalation is the separation (exfoliation) of the silicate layers by a convenient guest species (long alkyl chains). Two questions are interesting for the technology: (i) what is the optimum alkyl chain length and (ii) what is the optimum guest concentration for the preparation of precursor with the minimum exfoliation energy leading to the main goal: to obtain the polymer matrix with dispersed silicate layer plates. Silicate layer plates cause the hardening of polymeric material, which also become more thermally stable and more flame resistant. Combined structure analysis (modeling and powder diffraction) gave the answers for all the questions important for the technology. [6] Figure 2 shows the development of the structure of montmorillonite intercalated with octadecylamine in dependence on the guest concentration. (Small balls are the Na-cations, present in the interlayer space of the original host structure and compensating the negative layer charge, as the guest species are neutral molecules.)
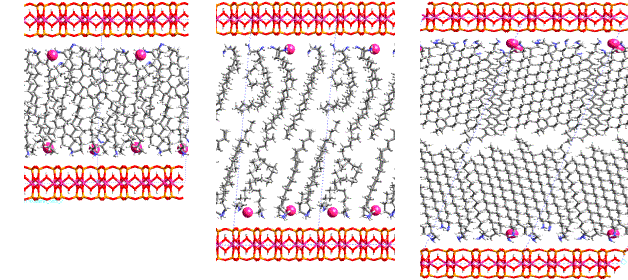

Figure 2
Acknowledgement
This study was supported by the Grant Agency of the Czech
Republic (GACR 202/03/0818 and GACR 205/02/0941).
[1] Ogawa, M., and Kuroda, K., Chem. Rev. 95, 399 (1995).
[2] Ogawa, M., Wada, T., and Kuroda, K., Langmuir 11, 4598 (1995).
[3] Lerf, A., in “ Handbook of Nanostructured Materials and Nanotechnology, vol. 5” (H. S. Nalwa, Eds.), p. 1. Academic Press, 2000.
[4] M. Pospíšil, P. Čapková, H. Weissmanová, Z.
Klika, M. Trchová, M. Chmielová, Z. Weiss, J.
Mol. Model 9, (2003) 39-46.
[5] Z. Klika , H. Weissmannová, P Čapková
and M. Pospíšil, Journal
of Colloid and Interface Science, In
Press, Available online 18 March
200
[6] M.
Pospíšil, P. Čapková, Z. Weiss, Z. Maláč, J. Šimoník, Journal of Colloid and Interface Science, 245 (2002) 126-132.