Crystal Structures of and Topological Aspects on the High Temperature Phases and the Decomposition Products of M2C2O4 M=[K, Rb,Cs]
Robert E. Dinnebier1, Sascha Vensky1,
Martin Jansen1, Jonathan Hanson2
1Max-Planck Institute for Solid State Research,
Heisenbergstrasse 1, D-70569 Stuttgart, Germany
2Chemistry Department, Brookhaven National Laboratory, Upton, NY
11973, U.S.A.
The high temperature phases of the higher homologues of the
alkali oxalates M2C2O4 M=[K, Rb, Cs] and their
decomposition products M2CO3 were investigated using fast
angle dispersive X-ray powder diffraction with an image plate detector and
DTA/DSC measurements. The following phases in order of decreasing temperature
have been observed and crystallographicaly characterized. A (*) denotes a
previously unknown modification: α-K2[C2O4]*, α-Rb2[C2O4]*
(Fig. 1),
α-Cs2[C2O4]*, α-K2[CO3],
α-Rb2[CO3]* (Fig. 1), α-Cs2[CO3]*: P63/mmc; β -K2[C2O4]*, β -Rb2[C2O4]*
(Fig. 1),
β-Cs2[C2O4]*, β-Rb2[CO3]*
(Fig. 1),
β-Cs2[CO3]*: Pnma;
β-K2[CO3]: C2/c; γ -K2[C2O4],
γ -Rb2[C2O4] (Fig. 1), γ -Cs[C2O4],
γ -K2[CO3], γ -Rb2[CO3]
(Fig. 1),
γ -Cs2[CO3]: P21/c; δ-K2[C2O4],
δ-Rb2[C2O4] (Fig. 1): Pbam.
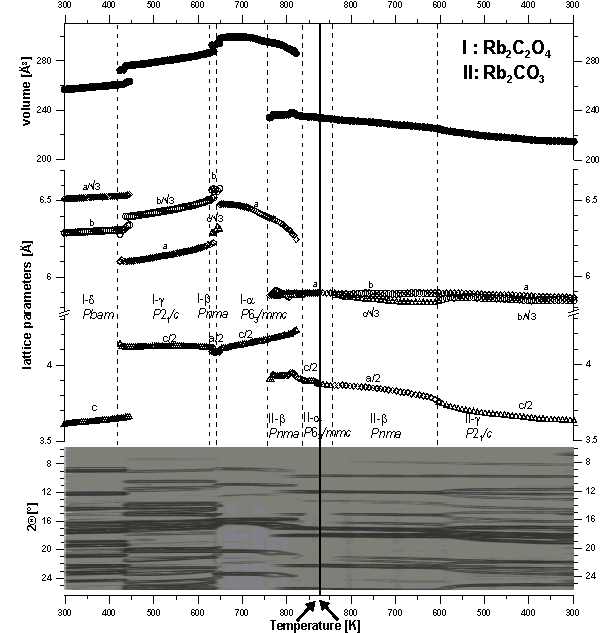
Figure 1
With respect to the center of gravity locations of the oxalate respectively carbonate dianions, the crystal structures of all known alkali- oxalates and – carbonates belong to the AlB2 structure family, crystallizing either in the AlB2 or in the Ni2In structure type. Despite the different sizes of the carbonate- and oxalate dianions the high temperature phases of the alkali carbonates M2CO3 M=[K, Rb, Cs] show the same sequence crystal structures as the corresponding (isotypic) alkali oxalates. Topological aspects and order-disorder phenomena at elevated temperature are discussed. For data reduction and graphical visualization of large sets of powder patterns, a new software POWDER3D has been developed which is subject of another abstract.
Acknowledgments:
This work was supported by the MPG, the FCI and the BMBF.