Synchrotron
radiation XRPD study of non ideality, thermal expansion and
Incommensurate-Normal structure phase transition in melilites
Marco
Merlini1,
Mauro Gemmi1, Gilberto Artioli1
1Dipartimento di Scienze della Terra
"A. Desio", Università degli Studi di Milano
Melilites are a
group of minerals which form continuos solid solutions between the end members gehlenite
(Ge, Ca2Al2SiO7) akermanite (Ak, Ca2MgSi2O7),
soda-melilite (NaMel, NaCaAlSi2O7) and Fe-bearing end members (Fe2+-gehlenite
– Fe3+-gehlenite / Fe2+-akermanite – Fe3+-akermanite).
The importance of these minerals is due to the fact that almost pure terms of
the series gehlenite-akermanite are among the first silicates which condensed
from the solar nebula and they are found in chondritic meteorites. Melilites
crystallise also in alkaline magmatic roks, whose origin is restricted to significant
geodynamic environments. Therefore a modelling of their thermodynamic
parameters is mandatory in order to improve the accuracy of phase equilibria
calculations, which could have important implications in petrological studies (i.e. for accurate determination of the
thermal history of meteorites, of the temperature of solidification of alkaline
roks ...). The thermodynamic calculations involving phase equilibria of
melilites are usually performed assuming the ideality of the solid solution
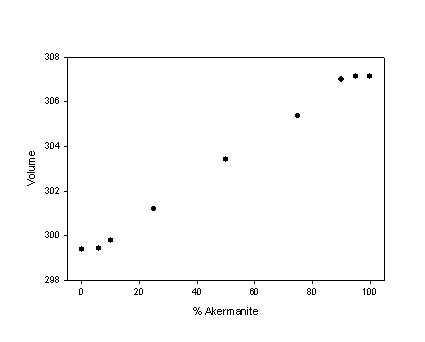
(s.s.) [1]. Nevertheless melilite s.s. has not an ideal behaviour (fig. 1). Our
results show that the unit cell volume curve in the ge-ak join has a sigmoidal shape,
with a negative and a positive excess molar volume close to ge and ak end-members
respectively. This is the general
behaviour of non equivalent site substitution mentioned by Newton and Wood [2]
for binary silicate solid solutions. An other important feature of melilites is
the presence of an incommensurate modulated (IC) structure, especially for
ak-rich compositions, which transforms into a normal (N) one upon heating [3,4,5].
The temperature of IC-N phase transition is of 80 °C for pure akermanite and it
is increased by Fe/Mg substitution and decreased by Al /(Mg+Si) exchange. We
performed high temperature (HT) X-ray powder diffraction (XRPD) on terms of
Ge-Ak-NaMel on the italian CRG beamline BM08 (GILDA) at ESRF, Grenoble in order
to measure reliable thermodynamics data (volume, thermal expansion) for several
composition and to investigate the IC-N phase transition for some terms near Ak
end-member. The experiments were performed with monochromatic radiation; the
samples were contained in quartz glass capillary, heated with a hot gas blower and
data were collected with a translating image plate (T.I.P.) [6]. IC and N
phases are marked by a strong difference in thermal expansion along the main
crystallographic directions. Thermal expansion measurements allowed to investigate
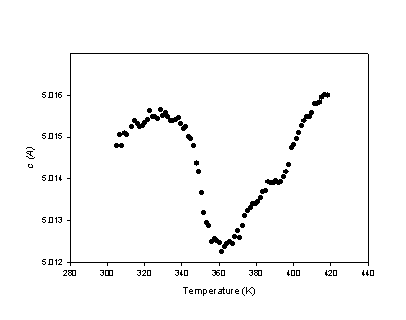
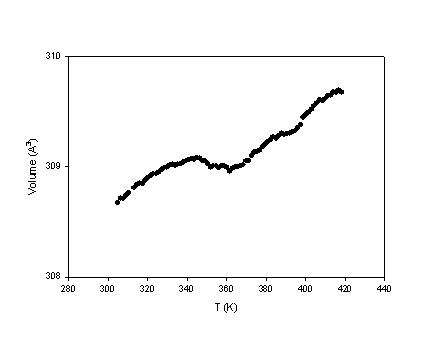
the field stability of IC phase near ak composition. The phase transition is
characterised by a contraction along c
and also a slight negative volumetric expansion (fig. 2-3). The results obtained
comprise also accurate thermal expansion measurements and crystal-structure
Rietveld refinements for melilites in Ak-Ge and Ak-NaMel joins in the
temperature range 270-1300 K.
|
|
|
|
Fig. 1 - Volume (Å3) in the join Ge-Ak at 300 K with the characteristic S-shaped form of the volume curve in binary silicate solid solutions. |
Fig. 2 - Variation of c lattice parameter value across the phase transition for akermanite measured with T.I.P. detector at GILDA beamline (ESRF) |
|
|
|
Fig. 3 - Thermal expansion of akermanite across the IC-N phase transition |
[1] Yoneda S. & Grossman L., 1995, Geoc. Cosmoch. Acta, 59, (16), 3413-3444
[2] Newton R.C. & Wood B., 1980, Am. Mineral., 65, 733-745
[3] Seifert et al., 1987, Phys. Chem. Minerals, 14, 26-35
[4] Rothlisberger et al., 1990, Eur. J. Mineral., 2, 585-594
[5] Yang et al., 1997, Phys. Chem. Minerals, 24, 510-519
[6] Meneghini et al., 2001, Journ. Synchrotron. Rad. 8, 1162-1166