HIGH TEMPERATURE BORATE CRYSTAL CHEMISTRY
S. Filatov1, R. Bubnova2
1Dept. of Crystallography, St.
Petersburg State University, University Emb. 7/9, St. Petersburg 199034, Russia
2Institute of the Silicate Chemistry
of Russ. Acad. of Sci., Ul. Odoevskogo, 24/2, St. Petersburg 199155, Russia
Thermal expansion of more than 40 borates has been investigated by powder X-ray diffraction and 30 of them demonstrate greatly anisotropic thermal expansion (αmax/αmin³ 5), moreover, about 20 of them show negative linear thermal expansion. The average linear coefficient of thermal expansion is about 25·10-6 oC-1 over 40 borates [1].
Role cations. When the borate thermal expansion coefficients are compared with chemical composition it is apparent that the average linear coefficient of thermal expansion rises with cation size increasing and cation valence decreasing.
High-temperature crystal structure investigation. To understand the sharply anisotropic character of borate thermal expansion we have studied crystal structures of some borates (α-CsB5O8, α-Na2B8O13 [2], LiB3O5 and Bi4B2O9) using single crystal high-temperature X-ray diffraction method. The main result is that boron-oxygen tetrahedra and triangles and rigid groups consisting of these B-O polyhedra do not change practically their configuration on heating but they can be turned of each other [1,2].
Role B-O-anions. The thermal structural behaviour of rigid groups provides an explanation of anisotropical character of thermal expansion for most borates. The B-O rigid groups do not essentially change their configuration on heating but they can be rotated relative to each other as hinges. In this case a large expansion along some directions is coupled with a contraction along others as it occurs in chain, layer and framework pentaborates. It is notable that existing of screw 21-chains of rigid B-O groups leads to greatly anisotropic thermal expansion of borates under study whereas insignificant influence of the B-O anion dimensionality on anisotropy of thermal expansion is revealed. As example all of the B-O anions in pentaborate structures (Figure) contain the same chains of the pentaborate groups extended along the 21 screw axis and all of them have the same maximum coefficient of thermal expansion along chains equal to about 60·10-6 oC-1 and negative one in the perpendicular direction equal to about -5·10-6 oC-1.
The highly anisotropic character of expansion of the structures based on finite complexes, e. g. Na2[B4O5(OH)4]•8H2O borax, may be rather caused by shear deformations.
Conclusion. To summarize, most borates demonstrate highly anisotropic character and great magnitude of thermal expansion: the average linear or volume coefficient of thermal expansion depends on size and valence of cation, whereas thermal behaviour of rigid BO groups dictates an anisotropy of thermal expansion.
The research has been supported by RFBR (project #
02-03-32842).
1. S.K.Filatov, R.S.Bubnova, Phys. Chem. Glasses 41 (2000) 216.
2. R.S. Bubnova, Yu.F. Shepelev, N.A. Sennova, S.K. Filatov, Z. Krist. 217 (2002) 444.
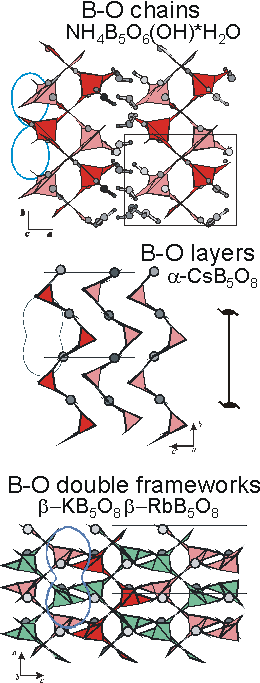
Figure. Hinge thermal expansion of pentaborates of 1-, 2- and 3-dimensionality.