The crystal structure of aromatic molecules under high pressure
M. Oehzelt1, G. Heimel1, A.
Aichholzer1, P. Puschnig2, K. Hummer2, C.
Ambrosch-Draxl2, M. Hanfland3, F. Porsch4, A.
Nakayama5, R. Resel1
1Institute of Solid State Physics,
Graz University of Technology, Petersgasse 16, A-8010 Graz, Austria.
2Department for Physics - Theoretical
Physics, University of Graz, Universitätsplatz 5, A-8010 Graz, Austria.
3European Synchrotron Radiation
Facility, BP 220, F-38043 Grenoble, France.
4Institute of Mineralogy and
Petrology, University of Bonn, Poppelsdorfer Schloß, D-53115 Bonn, Germany.
5Reserach Center for Advanced Carbon
Materials, National Institute of Advanced Industrial Science and Technology,
Tsukuba, Ibaraki 305-8565, Japan.
In the last
three decades, π-conjugated hydrocarbon materials have attracted a lot of
attention. In particular, organic polymers seem to be promising candidates for
low-cost, easy-processing materials for electro-optical and electronic
applications. Significant insight into many properties of these materials can
often be gained by studying a single, isolated molecule (e.g. in solution).
Nevertheless, for transport phenomena determining the performance of
(opto)electronic devices, intermolecular interaction in terms of wavefunction
overlap and low frequency (external) phonons will play a crucial role.
Moreover, important optical features such as the luminescence quantum yield in
the solid state can drastically differ form that of single molecules. Last but
not least, the strong anisotropy of the conductivity and the dielectric
function often found in crystals of short polycyclic organic molecules is
closely related to the specific way of molecular packing. Hence, a detailed
understanding of the crystal structure and the arrangement of the molecules
relative to each other is a prerequisite for understanding important bulk and
thin film properties in this classes of materials.
A deeper
insight into the nature of the intermolecular interactions and the packing
forces acting between the molecules can be gained by modulating the
intermolecular distances. Applying pressure to the sample is a ‘clean’ way to
tune the degree of intermoleculear interaction. There are essentially two
different kinds of atom-atom interaction in molecular crystals. On one hand
there are strong, covalent intramolecular bonds and, on the other hand, there
are weak, van der Waals-type forces acting between separate molecules. This
fact justifies to regard the molecules as rigid. Applying pressure brings the
whole molecular units closer together and/or changes their arrangement relative
to each other.
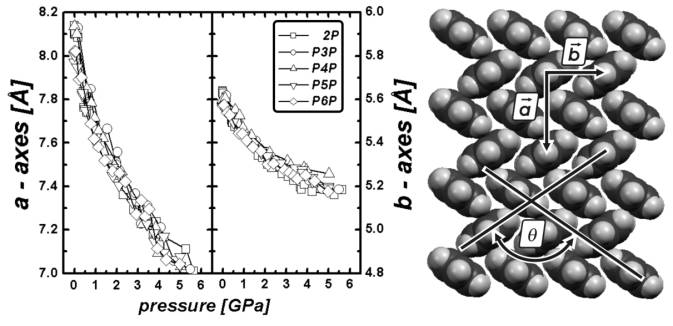
Figure 1: Lattice parameter a (left
panel) and b (right panel) of the oligo(para)-phenylenes
containing two to six phenyl rings as a function of hydrostatic pressure. The
lattice parameters of all oligomers have the same length at ambient conditions
and show the same pressure dependence. The left and right y axis cover the same
range (1.2 Å). Note that the lattice constant a is reduced
approximately twice as much as the lattice constant b in this pressure
region. The pictogram on the right side shows a projection of the crystal
structure of the oligo(para)-phenylenes vizualizing the two lattice
parameters a, b and the herring bone angle q.
This work is a
summary of measurements performed over several years. The samples for all
measurements were crystalline powders. The considered materials are the
oligo-acenes (anthracene - C14H10, tetracene - C18H12,
pentacene - C22H14), the oligo-phenylens (from biphenyl -
C12H10 to hexaphenyl - C36H26),
fluorene - C13H10, and perylene - C20H12.
Anthracene was measured at the photon factory BL18C (Tsukuba, Japan)[1,2], the
whole series of oligo-acenes and oligo-phenylenes at Hasylab BL F3 (Hamburg,
Germany)[3,4], fluorene and perylene at ESRF ID 9 (Grenoble, France) [work in
progress]. All experiments, except at the Hasylab BL F3 which is an energy
dispersive x-ray diffraction (EDXD) beam line, are done on angle dispersive
x-ray diffraction (ADXD) beam lines. The advantage of ADXD over EDXD is the
amount of information that can be drawn
from the diffraction data. In case of ADXD, the diffraction data could be
refined with the Rietveld method and in addition to the change of the lattice
constants, the rearrangement of the molecules (considered as rigid bodies)
within the unit cell could be determined. Pressures up to 22 GPa were applied
using diamond anvil cells (DAC).
In case of
these soft organic materials it is of major importance to guarantee appropriate
hydrostatic conditions. For example anthracene undergoes a phase transition if
it is not isotropically compressed [5], but it does not show a crystallographic
phase transition up to at least 22 GPa under hydrostatic conditions. In the
case of fluorene we observe a phase transition at 3.5 GPa under hydrostatic conditions
[paper in preparation]. The structure remains in the orthorhombic space group
but the angle of the herringbone pattern q (see
figures 1, 2) decreases significantly and an almost p-stacked regime is formed. The
herringbone angle changes from around 120° to around 60° during the transition,
while any compression in a non-hydrostatic environment results in a phase
transition at even lower pressures.
Figure 1 shows
the summary of changes in the ab-plane of the oligo-penylenes obtained
by EDXD measurements. Note that the lattice constant a changes twice as
much as the lattice constant b. This behaviour results in an effective
rotation of the molecules to higher herringbone angles q which is similar for all
oligo-phenylenes. The same behaviour of a changing twice as much as b
is shown in the oligoacenes. ADXD measurements of anthracene reveal more
details in the changing of the molecular packing. The rearrangement of the
molecules under pressure has a high impact on the band structure as well as on
the optical properties [4,6]. For example a redshift and a broadening of the
optical transition under pressure is observed, which has a purely
intermolecular origin.
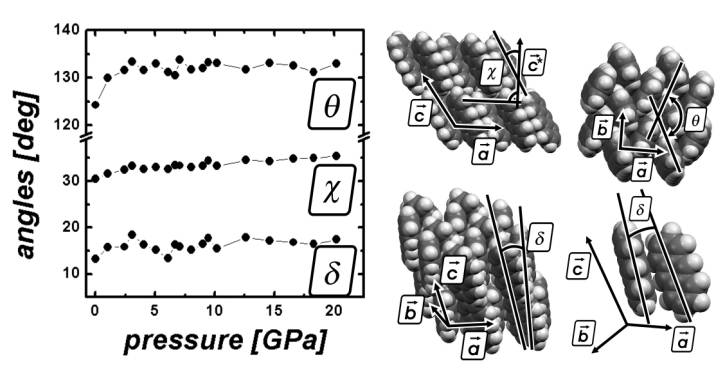
Figure 2: Rearrangement of the anthracene
molecules under pressure visualized by the angles q, c, and d. q is the herringbone angle and defined
as the angle between the molecular planes of the two translationally
inequivalent molecules. The setting angle between the long molecular axis and
the c* axis is denoted by c, whereas the tilt angle between the two long molecular axes of
translational inequivalent molecules is given by d.
Acknowledgement
This
research project is supported by the Austrian Science Fund (Project No.
P15626-PHY). M.O. also likes to acknowledge the Austrian Research Society
(ÖFG).
[1] M. Oehzelt,
R. Resel, A. Nakayama, Phys. Rev. B 66 (2002) 174104.
[2] M. Oehzelt,
G. Heimel, R. Resel, P. Puschnig, K. Hummer, C. Ambrosch-Draxl, K. Takemura, A.
Nakayama, J. Chem. Phys. 119 (2003) 1078.
[3] G. Heimel,
P. Puschnig, M. Oehzelt, K. Hummer, B. Koppelhuber-Bitschnau, F. Porsch, C.
Ambrosch-Draxl, R. Resel, J. Phys.: Condens. Matter 15 (2003) 3375.
[4] P. Puschnig,
K. Hummer, C. Ambrosch-Draxl, G. Heimel, M. Oehzelt, R. Resel, Phys. Rev. B 67
(2003) 235321.
[5] R. Resel, M.
Oehzelt, K. Shimizu, A. Nakayama, K. Takemura, Solid State Comm. 129
(2004) 103.
[6] K. Hummer,
P. Puschnig, C. Ambrosch-Draxl, Phys. Rev. B 67 (2003) 184105; Phys. Rev. Lett. 92 (2004)
147402.