Powder Diffraction Study of Thermal Expansion and Crystal Structure of the Aurivillius Phases in the Bi4Ti3O12–BiFeO3 System
M. Krzhizhanovskaya1,
S. Filatov1, V. Gusarov2, P. Paufler3, E.
Antoshchenkova1, R. Bubnova2, M. Morozov2, D.
Meyer3
1Dept. of Crystallography, St. Petersburg State University, University Emb. 7/9, St. Petersburg 199034, Russia
2Institute
of the Silicate Chemistry of Russ. Acad. of Sci., Ul. Odoevskogo, 24/2, St. Petersburg 199155, Russia
3Institut für Strukturphysik, Technische Universität Dresden, D-01062, Dresden, Germany
The Aurivillius family of layered Bi-containing oxides is well known for its ferroelectric properties. Usually their crystal structure is described as a combination or intergrowth of (Bi2O2)2+ layers and (An-1BnO3n+3)2- perovskite–like blocks, where A is a twelve co-ordinated cation e.g. Na, K, Ca, Sr, Ba, Pb, Bi, etc. and B is an octahedral cation such as Fe, Ti, Nb, Ta, Cr, etc.
In the Bi4Ti3O12 – BiFeO3
system the compounds with general chemical formula Bi2Bin-1(Ti,Fe)nO3n+3,
n = 3, 3.5, 4, 4.5, 5, 6, 8 synthesised by solid state reaction are noted in
[1]. The crystal structures of the compounds with n = 3, 3.5, 4 are determined
in orthorhombic system in [2], [3], [4] respectively. The structure of Bi4Ti3O12
(n=3) is refined by Rietveld method in orthorhombic space group B2cb at 25, 500
and 650 oC and in tetragonal space group I4/mmm at 800 oC
[5]. For Bi5Ti3FeO15 (n=4) the polymorph phase
transition from polar orthorhombic A21am space group to tetragonal
I4/mmm is studied in details by Rietveld method using PND data [6]. 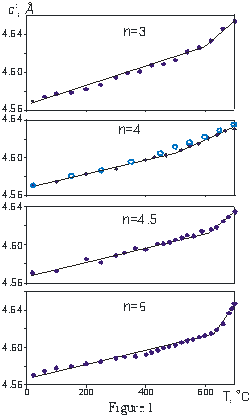 Its
transition temperature determined as ~ 730 oC is coincident with
Curie temperature corresponded to ferroelectric-paraelectric transition.
Its
transition temperature determined as ~ 730 oC is coincident with
Curie temperature corresponded to ferroelectric-paraelectric transition.
Now we investigate the thermal behaviour of four aurivillius phases with n = 3, 4, 4.5 and 6 using high-temperature X-ray powder diffraction in air (CuKa-radiation, the temperature range 20-700 oC) and DTA method (20-1300 oC). The temperature dependence of c cell parameter counting on a perovskite layer (c’) is presented in Fig. 1 along with the data from [6] for n=4. The dependence is obviously divided into two ranges: the low temperature region with a lower thermal expansion and a high temperature region with a greater expansion. The linear thermal expansion coefficient ac for HT phase is 3 times larger than that one for LT phase. Since a and b cell parameters (5.46+0.03 Å) and correspondingly the paired hkl, khl reflections are very close to each other in the studied compounds, it is complicated to calculate a and b values correctly. To define the orthorhombic-tetragonal transition point we analyze the FWHM temperature changes for unique and paired reflections. For example for Bi5(Ti,Fe)4O15 (n=4) the FWHM of the reflection hkl : 119 (I/Io=100%) practically does not change under heating: 0.33 o2q at 20 oC and 0.31 at 640 oC. FWHM of paired reflection 200 and 020 (I/Io=30%) decreases from 0.45 o2q at room temperature up to 0.31 at 640 oC. If the orthorhombic structure transfers to tetragonal the FWHM of unique and paired reflections becomes equal. Also a weak endothermic effect is observed on the DTA curve in the range of temperatures 650-740 oC in all studied samples. The Table 1 contains the following specific temperature points: (1) the inflection point of c cell parameter vs. temperature; (2) the temperature point in which the FWHM of unique and paired reflections becomes equal; (3) the DTA endothermic effect. Clearly, that the temperature of DTA effect is always higher than the changes in XRD pattern on heating. It could be caused by a different heating rate.
Table 1.
Thermal behaviour of aurivillius phases from HTXRD and DTA data. Specific
temperature points (oC): (A) the inflection point of c cell parameter vs. temperature; (B)
FWHM of paired peaks is equal to that of unique; (C) DTA endothermic effect
|
|
A |
B |
C |
|
n=3 |
610 |
660 |
660 |
|
n=4 |
500 |
640 |
740 |
|
n=4.5 |
600 |
600 |
690 |
|
n=6 |
620 |
620 |
650 |
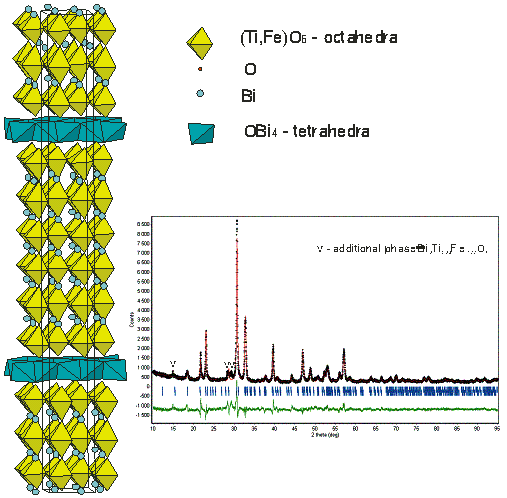
As far as we are aware, there is only one crystal structural study of Aurivillius phase structure with c parameter 57.6 Å corresponded to a 6 layer-structure [7]. For Bi7Ti3Fe3O21 we propose the structural model based on 6 layered perovskite blocks. To build up the model the atomic coordinates of Bi5Ti3FeO15 [4] were used. It was refined in orthorhombic Fmm2 space group: a=5.474(1), b=5.495(1), c=57.60(1) Å with Rietica for Rietveld refinement program (Rp = 10.2, Rwp = 13.3, RB=5.6%). It was also refined in A21am space group but no additional reflections which are compatible with this lower symmetry were observed. Relatively high R-factors are caused probably by the structure defects. The FWHN for reflection of 00l type is two-three times larger than that one for other reflections. Perhaps the number of layers in block is not constant in the structure, but the considerable amount of 6-layer phase is present. This structure could also be described in terms of crystal chemistry with oxocentered anions as an alternation of perovskite blocks and Bi-O layers build up from OBi4 tetrahedra by sharing edges (Figure 2).
Figure 2. The Crystal structure of Bi7Ti3Fe3O21 and the Rietveld data plot.
1. M.I. Morosov, V.V. Gusarov, Inorg. Materials 38 (2002) 723-729
2. G.N. Subbanna, T.N. Guru Row, C.N.R. Rao, J. Solid State Chem. 86 (1990) 206-211
3. B. Aurrivillius Ark. Kemi. 1 (1949) 463-471, 499-512, 519-528
4. C.H. Hervoches et al. J. Solid State Chem. 164 (2002) 283-286
5. C.H. Hervoches and P. Lightfoot, Chem. Mater. 11 (1999) 3359-3364
6. A. Snedden, C.H. Hervoches, P.Lightfoot Phys. Review B67 (2003)
7. D. Mercurio, G. Trolliard, T. Hansenand, J. P. Mercurio, Int. J. Inorg. Mater. 2 (2000) 397-406