Temperature effects on the hydrogen bond patterns in some hydrated aminoacids
Asiloé J. Mora1, Edward Avila1, Gerzon
E. Delgado1, Andrew N. Fitch2, Michela Brunelli2,
Aira Miró3, Rafael Almeida3, Luis Rincón3
1Laboratorio de Cristalografía, Facultad de
Ciencias, Universidad de Los Andes, Mérida 5101, Venezuela
2European Synchrotron Radiation Facility, B.P. 220, F-38043 Grenoble Cedex, France
3Grupo de Química Teórica, Facultad de
Ciencias, Universidad de Los Andes, Mérida 5101, Venezuela
Cooperative effects are significant in the formation of extended patterns of hydrogen bonds in amino acids, specially when the stabilization is achieved by polarization and charge transfer effects [1,2]. On the other hand, the water molecule is unique, since it has the possibility of acting as a double donator and double acceptor of hydrogen bonds, playing an important role in biological processes such as folding and hydration of proteins [3,4]. It also helps to preserve their three-dimensional structures. If it is present in the crystal structure of small aminoacids, it can play an important role in the hydrogen-bonding patterns through cooperative effects, or reside in void spaces in the structure forming weak aminoacid-solvent interactions. In the hope of understanding the role played by the water molecules in simple biological systems, we have undertaking an investigation on the energetic and structure of hydrogen-bonding patterns of two structurally related aminoacids, 4-piperidine carboxylic acid and cis-4-aminocyclohexane carboxylic acid (Figure 1). The first has the potential to donate two hydrogen atoms for hydrogen bonding, while the second, having the animo group as a substituent of the cyclohexane ring, is able to donate three hydrogen atoms. Differential Scanning Calorimetry and Thermogravimetric Analysis were performed to investigate the dehydration process and any additional phase changes upon heating. Structural changes were followed by means of variable temperature X-ray powder diffraction experiments using beamlines BM16 and ID31, ESRF, France. Figure 2 shows a three-dimensional diagram for 4-piperidine carboxylic acid, in which three different crystalline phases are seen. In the case of cis-4-aminocyclohexane carboxylic acid only two phases were noticed. The different structures were solved by simulated annealing and refined using the Rietveld Method. In the 4-piperidine carboxylic acid, the motifs were extended chains in two crystallographic directions, while in the cis-4-aminocyclohexane carboxylic acid sandwich structures, formed by two amino acid units linked by head-to-tail hydrogen bonds, are the basic motif. These sandwich structures then form extended helicoidal chains. A detailed discussion of the hydrogen-bonding patterns of the hydrated, dehydrated and high temperature forms of the amino acids is presented, and compared with semi-empirical theoretical calculations performed in these systems. From our investigations we conclude that the water molecule has the additional role of completing the acceptor capacity of the carboxylate group of the amino acids, which is equal to four in the cases investigated here.
|
|
|
Figure 1. (a) 4-piperidine carboxylic acid (b) cis-4-aminocyclohexancarboxilic acid
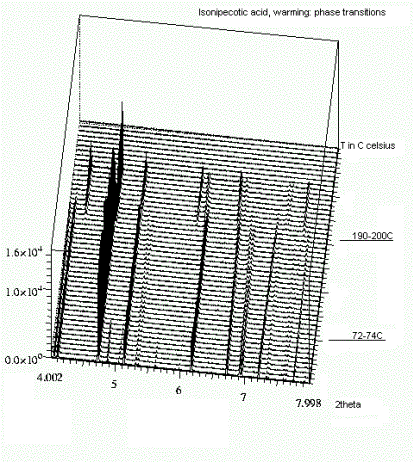
Figure 2. 3D Temperature dependent X-ray diffraction plot for 4-piperidinecarboxylic acid
Acknowledgement
This study was supported by the CDCHT-ULA, FONACIT
(Lab-97000821) and ESRF (France).
1. A.J.
Mora, G.E. Delgado, B.M. Ramírez, L. Rincón, R. Almeida, J. Cuervo, A. Bahsas. J.
Mol. Struc., 615 (2002) 201.
2. J. Cuervo, L. Rincón, R. Almeida, A.J. Mora, G.E. Delgado, A. Bahsas. J. Mol. Struc., 615 (2002) 191.
3. H. Roder and G. Elove. In mechanisms of protein folding (Ed R.H. Pain), Oxford Universtity Press, NY (1994).
4. Ch. Toyoshima, M. Nakasako, H. Nomura and H. Ogawa, Nature, 405 (2000) 647.