Structural Investigation of a New “Family” of Chiral 3D Nickel
Glutarates with Intersecting 20-Membered Ring Channels
N. Guillou, C. Livage, J. Chaigneau and G. Férey
Institut Lavoisier, UMR CNRS C8637, Université de Versailles Saint-Quentin-en-Yvelines, 45 Avenue des Etats-Unis, F-78035 Versailles, France
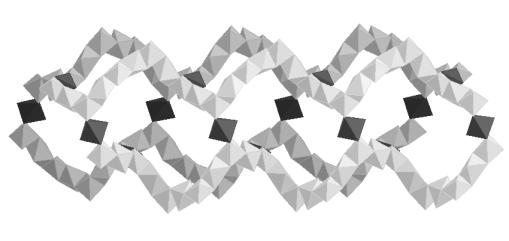
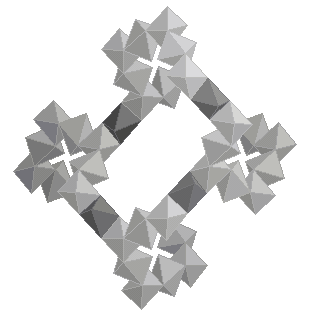
Nickel hybrids constitute an important new focus of research in material chemistry, offering potential applications in adsorption, catalysis, nonlinear optical devices and magnetic materials. The plasticity of nickel oxide condensation, which generates a wide range of structural edifices, has been demonstrated once again, by recent results on large pore zeolitic compounds (1, 2). Due to the difficulty to grow single crystals of nickel hybrids, powder diffraction is most of the time essential (3). We report here one of the most significant results on this topic, with the description of a series of nickel glutarates. Three compounds, Ni20(glutarate)20(H2O)8.40H2O (1), Ni20(3-methylglutarate)20(H2O)8.24H2O (2) and Ni20(2-methylglutarate)20(H2O)8.18H2O (3), were prepared as green powders from hydrothermal reactions. Their structures were solved ab initio from conventional X-ray sources or synchrotron powder data. All powder patterns were indexed in the cubic symmetry by using DICVOL91, with a similar unit cell parameter [a = 16.5812(7) Å, a = 16.75633(7) Å and a = 16.5419(3) Å for (1), (2) and (3), respectively]. Systematic extinctions were consistent with the two chiral space groups P 41 3 2 and P 43 3 2. All structures present the same amazing chiral three dimensional inorganic network of edge sharing nickel octahedra, generated by two independent nickel atoms located on the threefold and twofold axes. This complicated oxide network can be simply described from helices running along the a axis (Fig. 1). Each helix is connected to four out-of-phase parallel neighbouring ones through a nickel octahedron, generating corrugated twenty membered rings. That also induces the formation of perpendicular helices.
|
|
|
|
(a) |
(b) |
|
Fig. 1. (a) View of four
helices (light grey) connected by nickel octahedra (dark grey); (b) view of these four helices in the perpendicular
direction. |
|
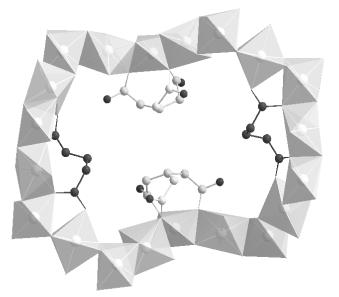
The oxide framework is decorated by two independent deprotonated organic anions (Fig. 2). The first one (dark grey) has two multidendate carboxylate groups which are each coordinated to three nickel atoms. The second one (light grey) presents two carboxylates with a m2 oxygen and a non bonded one and has a statistic occupancy of 2/3. Bridging water molecules complete octahedral nickels environments. The main difference between the three structures seems to concern this disordered organic anion. The twenty membered rings intersect each other to generate very large crossing tunnels in the [111] direction, which contain disordered water molecules.
|
|
|
Fig. 2. Polyhedral view of a corrugated twenty membered ring in structure of (1) with the two independent glutarate ions. The light grey one is disordered with statistic occupancy of 2/3. |
Thermal behaviour of nickel glutarate (1) has been studied by temperature dependent X-ray diffraction and thermogravimetric analysis. Disordered occluded water molecules leave the structure without significant structural change, to render the material porous after activation at 200°C under vacuum [346(10) m2 g-1]. The loss of the eight coordinated water molecules is combined to crystallographic changes observed at 240°C. Symmetry and space group are conserved with a contraction of the unit cell [a = 15.653(1) Å)]. Curiously, a drastic decrease of the porosity is observed, which corresponds to structural rearrangements, mainly of the organic moieties. Its total rehydration allows recovering the original structure and shows the flexibility of this topology, with a reversible breathing of the 3D nickel oxide network.
1. P. M. Forster and A. K. Cheetham Angew. Chem. Int. Ed, 2002, 41, 457.
2. N. Guillou, C. Livage, M. Drillon and G.
Férey Angew. Chem. Int. Ed, 2003, 42, 5314.
3. N. Guillou, C. Livage, W. van Beek, M. Noguès
and G. Férey Angew. Chem. Int. Ed, 2003, 42, 457.