X-ray powder diffraction used to evaluate the kinetics of various processes related to ordering in Ni1+dSn (d = 0.35, 0.50)
A. Leineweber, E.
J. Mittemeijer
Max Planck
Institute for Metals Research, Heisenbergstraße 3, 70569 Stuttgart, Germany.
The binary
system Ni-Sn contains a series of non-stoichiometric phases Ni1+dSn with Ni2In/NiAs type structures:
a NiAs type sub-structure is formed by Ni(1)Sn (Sn taking the place of As) with
additional Ni(2) atoms occupying partially the centres of the
trigonal-bipyramids formed by five Sn atoms. In the hexagonal high-temperature
(HT) phase with ca. 0.27 £ d £ 0.65 these Ni(2) atoms show no long-range order.
Occupational ordering of Ni(2) is exhibited, however, by the three different
but structurally similar orthorhombic low-temperature phases (LT, LT', LT''
[1-5]). The order-disorder phase-transition temperature is strongly composition
dependent, e.g. the disordered HT-Ni1.50Sn transforms to the ordered
LT phase below 782 K, whereas for Ni1.35Sn the LT’ phase forms below
686 K. The equilibrium phase transitions occur discontinuously, i.e. by a first
order transition [2]. States of (dis)order can be retained at ambient
temperatures by quenching the alloys in water.
The present
paper reports X-ray powder diffraction investigations on non-equilibrium states
of Ni1+dSn obtained by various heat
treatment procedures, providing insight into the mechanism of the different
observed processes. For that three alloys were equilibrated at different
annealing temperatures T1 and subsequently quenched:
HT-Ni1.50Sn
quenched from T1 = 1023 K
HT-Ni1.35Sn
quenched from T1 = 1023 K
LT’-Ni1.35Sn
quenched from T1 = 683 K.
These samples
were afterwards annealed at various temperatures T2 < 600
K for certain periods of time, quenched and then characterised by X-ray powder
diffraction. The gradual changes from the equilibrium state quenched from T1
to the new equilibrium state (often finally reached only after long times) at T2
can be traced by measuring lattice parameters, integral reflection intensities
and diffraction-line broadening as a function of the annealing time.
Annealing of
HT-Ni1.50Sn and HT-Ni1.35Sn at T2
generates new long-range order leading finally to the LT/LT’ phases in
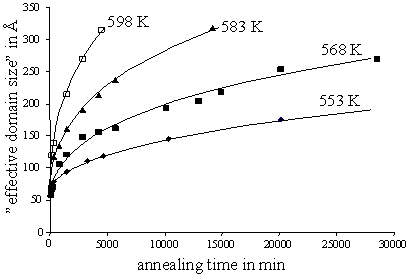
equilibrium at T2. This order formation occurs in a two-step
fashion, which involves firstly formation of long-range order in small domains,
and secondly coarsening of the ordered domains as exhibited by a narrowing of
the superstructure reflections (Figure 1).
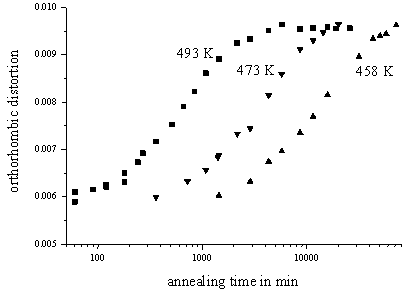
The LT’-Ni1.35Sn
sample equilibrated at and quenched from T1 = 683 K shows
long-range order with sharp superstructure reflections. However, the degree of
long-range order as obtained from the intensities of the superstructure
reflections is distinctly lower than for samples equilibrated at lower
temperatures. In this case annealing at various temperatures T2
now reveals simply a gradual increase of the already present long-range order
(Figure 2), in contrast with the two-stage order-formation process exhibited by
the samples equilibrated at T1 = 1023 K.
The X-ray
powder diffraction data were used to evaluate the kinetics of the observed
processes. Particularly, the activation energies were determined. Methods for
such analyses were developed. The contribution of the activation-energy values
to the understanding of the transformation mechanisms is discussed.
|
|
|
|
Figure 1: Coarsening of small-domain LT-Ni1.50Sn produced by annealing HT-Ni1.50Sn as observed by an evaluation of Scherrer-type broadening of the superstructure reflections (stage 2 of order formation). |
Figure 2 Evolution of the ‘orthorhombic distortion’ used as a measure for the degree of long-range order in LT’-Ni1.35Sn equilibrated at T1 = 683 K and annealed subsequently for various times at various temperatures T2. |
1. P. Brand, Z. Anorg. Allg. Chem. 353 (1967) 270-280.
2. H. Fjellvåg, A. Kjekshus, Acta Chem. Scand. A40 (1986) 23-30.
3. A.
Leineweber, M. Ellner, E.J. Mittemeijer, J. Solid State Chem. 159 (2001)
191-197.
4. A.
Leineweber, J. Solid State Chem. 177 (2004) 1197-1212.
5. A.
Leineweber, E. J. Mittemeijer, Z. Anorg. Allg. Chem. 628 (2002)
2147-2147.