Neutron diffraction study crystal structures of KAlO2 and Cs3PO4 in wide range of temperatures
Voronin V.I.a, Proskurnina N.V.a, Shekhtman G.Sh.b, Byrmakin E.I.b,
Stroev S.S.b
a Institute of Metal Physics, Ural Division of Russian Academy of Sciences,
S. Kovalevskoi Str.18, Ekaterinburg GSP-170, Russia
b Institute of High Temperature Electrochemistry, Ekaterinburg, Russia
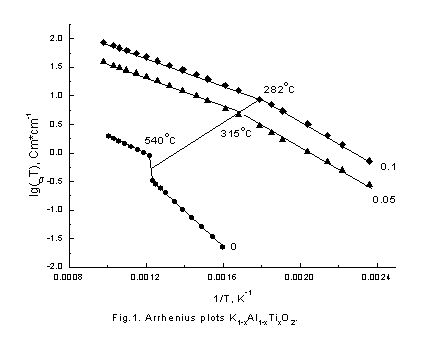
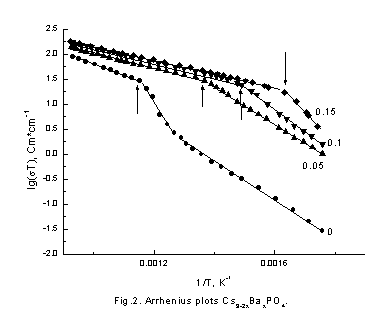
Solid orthophosphate ceria and potassium aluminate electrolytes are desirable materials for industrial applications. It is well known that the main factor determining an electrical property of the solid electrolytes is their crystal structure. Therefore, cation conductivity and crystal structure of KAlO2 and Cs3PO4 has been studied in present work. Conductivity dependences on temperature in Arrhenius plots – lg(sT)-vs 1/T are shown in Figure 1 and 2.
|
|
|
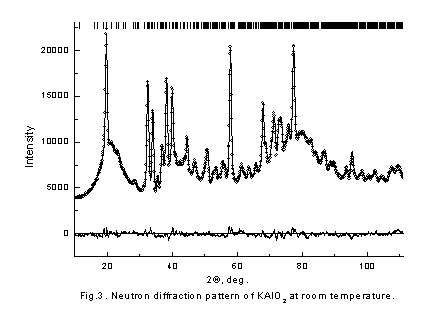
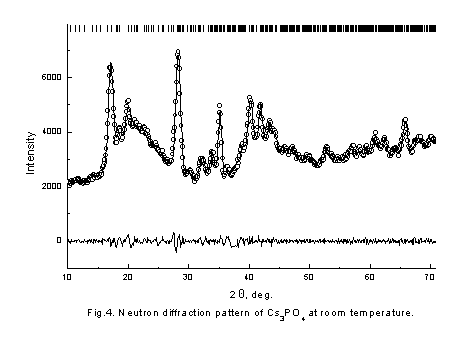
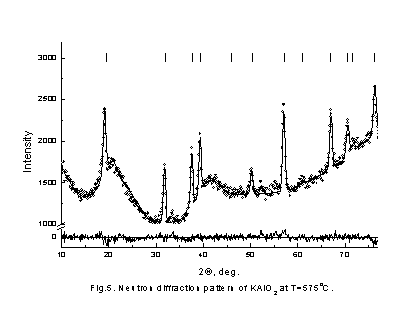
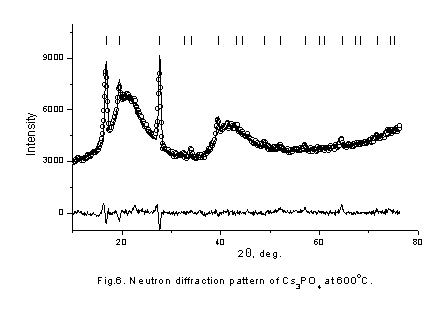
It is seen that behavior of conductivity is very similar in these compositions. In initial KAlO2 and Cs3PO4 samples conductivity jump are observed in high temperature region and the values of activation energy are decrease. The conductivities are significantly increased as the doped element content increases, end bending points are on Arrhenius plots at some temperatures. To understand the reason of such behavior of conductivity neutron-diffraction experiments in a wide range of temperatures were carried out. Because of samples chemical activity and their hygroscopicity and to prevent their contacts with air all samples were placed in quartz-closed ampoules. Therefore, there is a wide diffused halo of amorphous quartz on neutron diffraction patterns. The neutron diffraction patterns obtained for KAlO2 and Cs3PO4 (fig. 3,4) show that the samples were monophasic. The Rietveld analysis of the diffraction pattern revealed that the KAlO2 and Cs3PO4 crystallize in the orthorhombic space groups, Pbca for KAlO2 (a = 5.4446(9) Å, b = 10.931(1) Å, c = 15.458(2) Å) and Pmmm for Cs3PO4 (a = 14.590(5) Å, b = 10.219(3) Å, c = 7.780(2) Å). Analyze also showed that KAlO2 structure has the tetrahedra AlO4 forming three-dimensional framework with full connectivity. On the contrary in Cs3PO4 the tetrahedra PO4 are separated spatially. We found that Cs3PO4 and KAlO2 exhibits structural phase transition at T ~ 790 K and ~ 813 K, correspondingly (fig. 5,6), followed by the thermal conductivity jump (fig. 1,2).
|
|
|
Using high temperature structure b-cristobalite study
results [111] it has been showed that at Т>540oC the
aluminum, potassium and oxygen atoms occupy, respectively, the special
equivalent positions 8a, 8b and 16c in the space group Fd-3m.
It is an “ideal” model that gives a bad experiment description. Disordered
models give good accordance with experiments. In the first model the oxygen atoms do not lie on
well-defined positions, but the Al-O bond precesses about its average
orientation so that the oxygen atoms lie on an annulus of fixed radius (~0.5
Å). In the second
model the oxygen atoms are placed in the 96h sites, with partial
occupancy of 1/6. We cannot prefer any of
these models from our experimental data. More precise example is given in Fig.
5.
In high temperature phase orientationally disordered phosphate ions occupy an FCC lattice, and the sodium cations occupy all tetrahedral and octahedral interstices (neutron diffraction patterns show at fig.6).
|
|
|
Crystal structures of KAlO2 and Cs3PO4 are cubic at room temperature because of doping them by titanium and barium, respectively. However these cubic structures are disordered as for according high temperature phases, but this disordering is static and dynamic at high temperature. There is an increase of disordering degree of the K1-xAl2-xTixO2 (x=0.2) sample as the temperature increases and Debay-Waller factor significant increases.
Because of crystal structure features it was suggested the
conductivity mechanism at high temperatures in so called superionic state. As
it was mentioned earlier there are rigid tetrahedra PO4 groups in orthophosfat cesium which are saved
their form in whole temperature region. However, large Debye -Waller
factors in superionic state suggest dynamic disordering. These facts mean group
motion as a whole. Such model was suggested for isostructural compound Na3PO4 based on complex
study of structural state at high temperatures using different methods [666].
Inelastic neutron scattering experiments showed rotor motion of these
complexes. It’s the model
suggests strong coupling between the reorientation of anions and the mobility
of cations – the “paddle-wheel mechanism”. The cubic structure appears at room
temperature at doping of compounds that is why such correlated tetrahedra PO4 rotation and
cesium ions diffusion appears at lower temperature. This process is illustrated
by bending point on Arrhenius plots; bending point temperature decreases with
barium concentration (Fig.2).
Additional conducting channels appear at phase transition into
cubic phase in KAlO2 lattice. At the same time such “paddle-wheel mechanism” occurs in KAlO2.
Because of tetrahedrals link by oxygen
atoms there are correlating AlO4 vibrations and motion of K cations
with increasing temperature. This correlation is confirmed by sharp activation
energy decreasing at doping with titanium (Fig.1). And the bending point
temperature decreases as the Ti concentration increase.
Work supported by State Scientific Research Program “Neutron
Investigations of Condensed Matter” (State control No. 40.012.1.1.11.50) and
the basic research program of the Department of Physical Sciences of the
Russian Academy of Sciences "Neutron studies of the substance structure
and fundamental properties of matter”.
Swainston I.P. and Dove M.T., J.Phys.: Condens. Matter., 7 (1995) 1771.
Wilmera D., Feldmanna H., Combetb J., Lechner R.E., Physica B 301 (2001) 99