Complex Intermetallic Compounds in
the Mg-Ir System Solved by Powder Diffraction
,
aUniversity of Geneva, 24 quai
Ernest-Ansermet, CH-1211 Geneva 4, Switzerland
bCEA/Grenoble, 17, rue des Martyrs,
F-38054 Grenoble Cedex 9, France
In the Mg–Ir phase diagram only the composition interval 0–25 at. % Ir was investigated [1]. Nothing is known about compounds with higher iridium content. Recently, new compound with the composition of Mg5Ir2 (28.6 at. % Ir) was reported [2], and found to crystallize with the hexagonal Al5Co2 type structure. Here we report on two new topologically closed-packed intermetallic compounds rich in iridium with new structure types. Their crystal structures were fully characterized by high resolution synchrotron powder diffraction (SNBL, l ~ 0.5 Å, sample in a 0.2 mm glass capillary), global optimization of a structural model in direct space using the simulated annealing (in parallel tempering mode) using the program FOX [3] and refined by FullProf.2k.
MgIr:
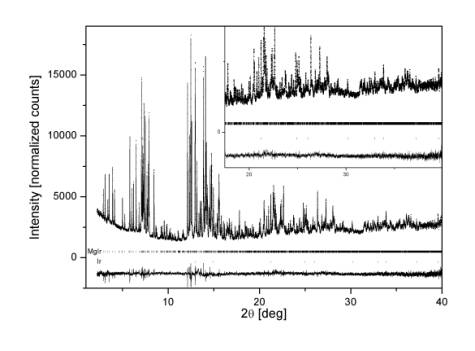
S.g. Cmca, 25 independent atoms, a = 18.46948(6), b = 16.17450(5), c = 16.82131(5) Å, 76 parameters, Rwp = 0.094, c2 = 3.02, RB = 0.056, measured composition (EDAX) Mg52(2)Ir48(2). The structural model was independently confirmed by the single crystal X-ray diffraction [4]. The Ir-Ir interatomic distances in MgIr are in the range of 2.424(4) - 2.667(2), and are the shortest ever observed in an Ir-containing compound.
|
|
|
|
Figure 1: Rietveld plot of MgIr. |
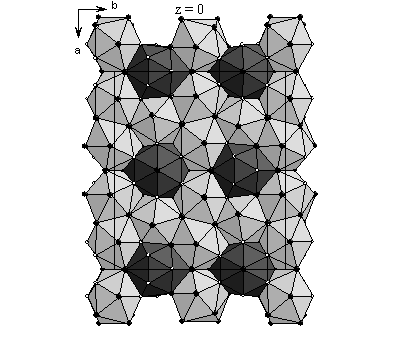
Figure 2: Structural slab of MgIr. Ligand atoms: Ir as small white and Mg as large black spheres |
.
Mg2Ir3:
S.g. C2/m, 11 independent atoms, a = 18.5700(2), b = 5.18716(3), c = 8.49240(6) Å, β=97.2211(5)°, 27 parameters, Rwp = 0.141, c2 = 1.44, RB = 0.053. The structure is derived from that of the hexagonal Laves phase MgIr2, which was also observed by us, by stacking of deformed MgIr2 blocks that are two IrMg12 icosahedra thick.
[1] Binary Alloy Phase Diagrams. (1996). Second Edition, version 1.0, ASM International.
[2] Černý, R., Joubert, J.-M.,
Kohlmann, H. & Yvon, K. (2002). J. Alloys Comp. 340, 180-188.
[3] Favre-Nicolin, V. and Černý, R., J. Appl. Cryst. 35 (2002) 734-743 see also: http://objcryst.sourceforge.net
[4] Černý, R., Renaudin, G., Favre-Nicolin, V., Hlukhyy, V. and Pöttgen, R. Acta Cryst. B, in press.